What is the atomic number of Antimony?
49
51
53
55
Dive into the intriguing world of Antimony with our comprehensive guide, crafted especially for educators. Antimony, a lesser-known yet significant element, holds a unique place in both science and history. As a teacher, understanding its complexities and conveying them to students can be challenging. Our guide simplifies this task, offering clear explanations, practical examples, and engaging teaching tips. Enhance your lessons and captivate your students with our in-depth exploration of Antimony.
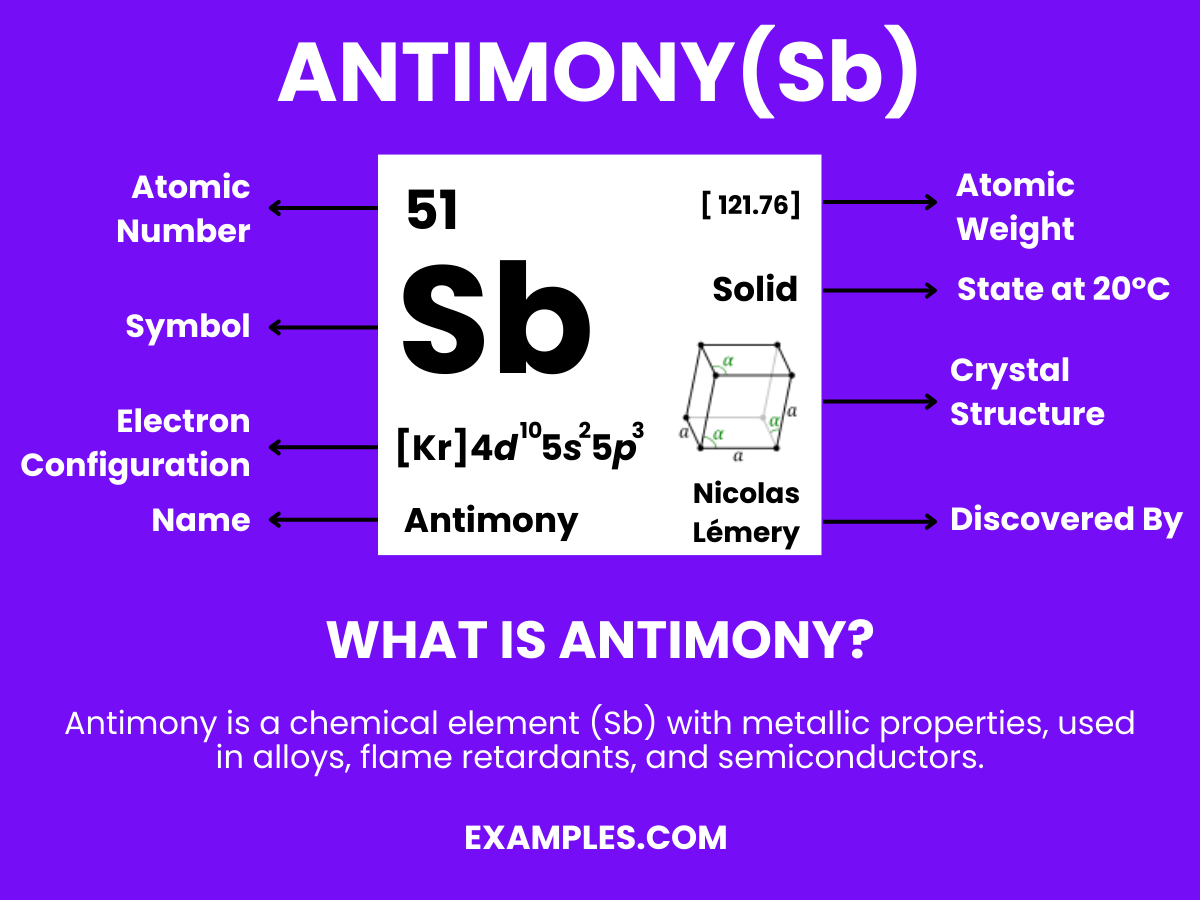
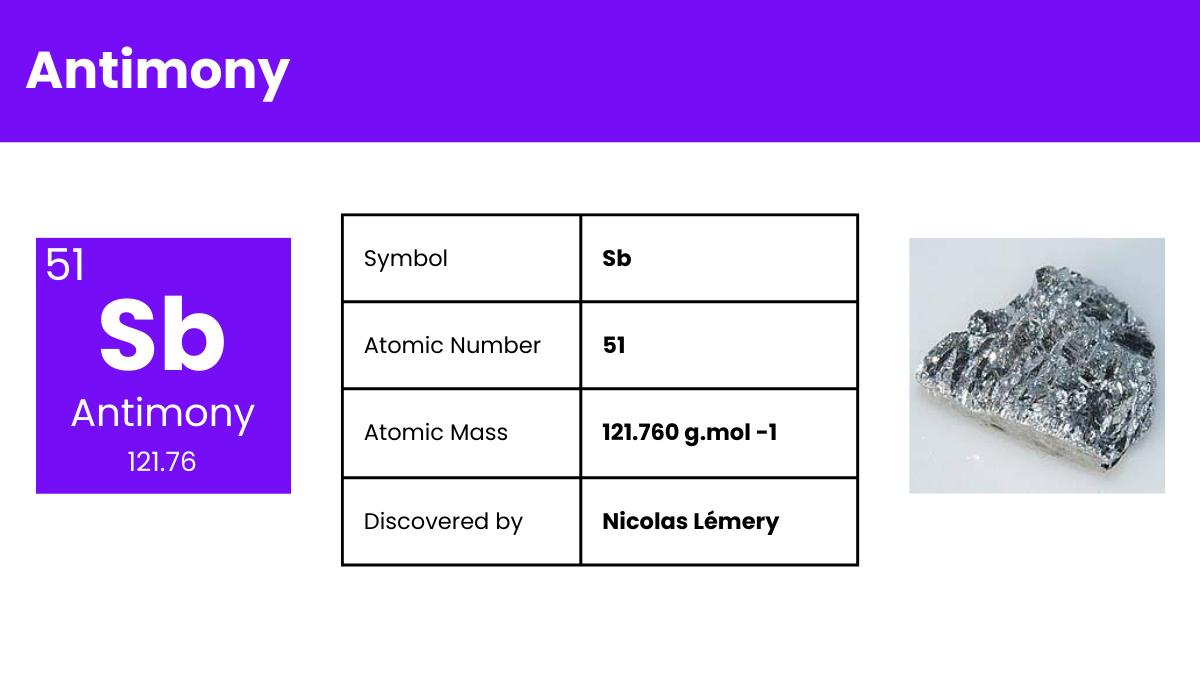
Antimony is a chemical element with the symbol Sb (from Latin: stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient times and were used for cosmetics; metallic antimony was also known, but it was erroneously identified as lead upon its discovery. The largest applications for metallic antimony are as alloying material for lead and tin and for lead antimony plates in lead-acid batteries. Alloying lead and tin with antimony improves the properties of the alloys which are used in solders, bullets, and plain bearings.
| Boron (B) |
| Silicon (Si) |
| Germanium (Ge) |
| Arsenic (As) |
| Tellurium (Te) |
| Property | Description |
|---|---|
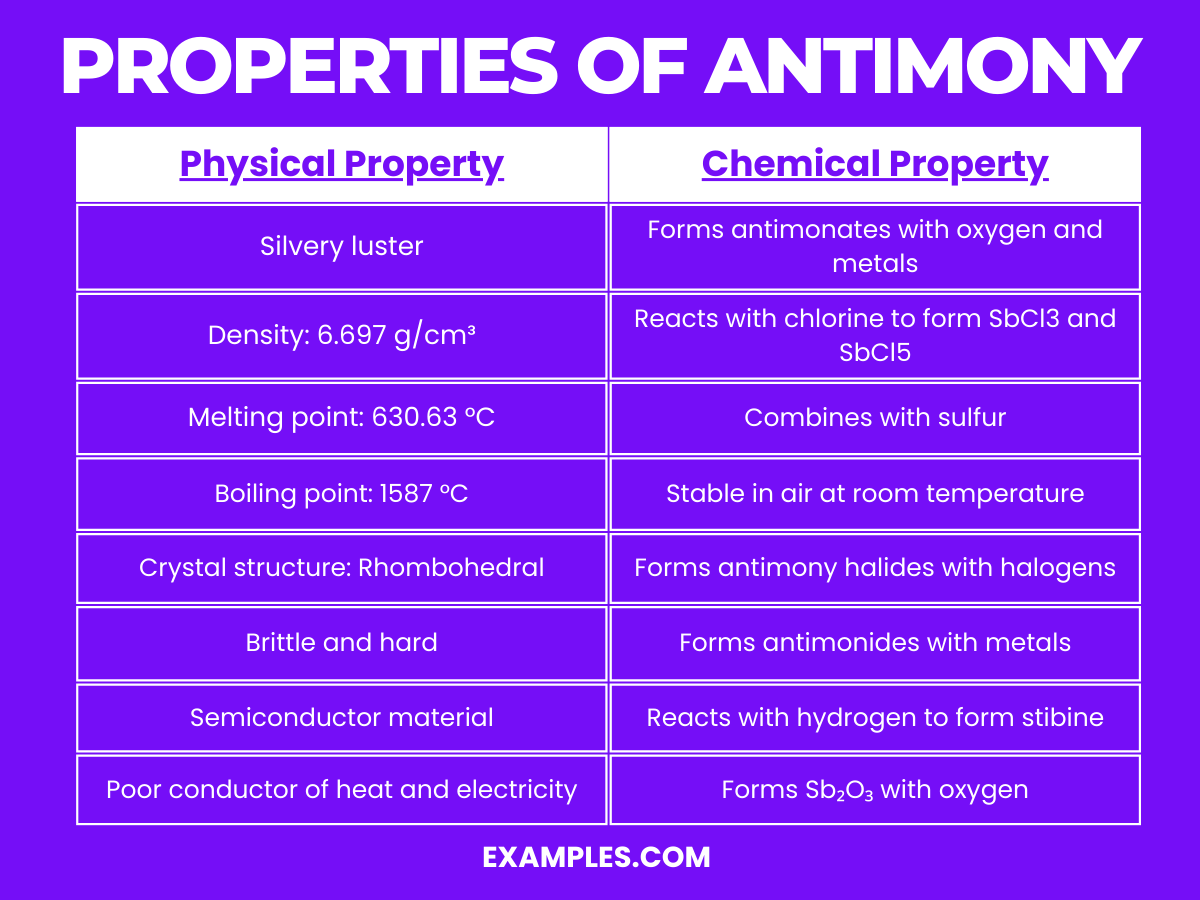
| Appearance | Silvery, lustrous gray metalloid, exhibiting a metallic shine. |
| Density | Approximately 6.697 g/cm³, indicating a relatively high density. |
| Melting Point | Melts at about 630.63 °C, showing moderate thermal stability. |
| Boiling Point | Boils at 1587 °C, indicative of its high thermal endurance. |
| Crystal Structure | Exhibits a rhombohedral crystal structure, contributing to its brittleness. |
| Hardness | Fairly hard, though brittle, making it difficult to machine or work with. |
| Conductivity | Poor conductor of heat and electricity, useful in certain alloys and compounds. |
| State at Room Temperature | Solid under standard conditions, maintaining its structure without degradation. |
Antimony, with the chemical symbol Sb, exhibits several interesting chemical properties:
| Property | Description / Value |
|---|---|
| Melting Point | 630.63°C (1167.13°F) |
| Boiling Point | 1587°C (2889°F) |
| Thermal Conductivity | 24.4 W/(m·K) |
| Specific Heat | 0.207 J/(g·K) at 25°C |
| Heat of Vaporization | 195.2 kJ/mol |
| Heat of Fusion | 19.79 kJ/mol |
| Property | Description / Value |
|---|---|
| Phase at STP | Solid |
| Density | 6.697 g/cm³ at 20°C |
| Young’s Modulus | 55 GPa |
| Tensile Strength | 170 MPa |
| Mohs Hardness | 3 |
| Elastic Modulus | 55 GPa |
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Diamagnetic |
| Electrical Conductivity | 2.5×10^4 S/m at 20°C |
| Property | Description / Value |
|---|---|
| Atomic Number | 51 |
| Atomic Mass | 121.760 u |
| Neutron Cross Section | 5.6 barns (for ^121Sb) |
| Isotopes | ^121Sb (57.21%), ^123Sb (42.79%) |
| Radioactivity | Natural antimony is stable. ^125Sb and ^124Sb are notable radioactive isotopes with half-lives of 2.75856 years and 60.20 days, respectively |
Antimony forms several important compounds, each with unique properties and applications. Here are the top six antimony compounds along with their relevant chemical equations:
| Isotope | Key Characteristics |
|---|---|
| ¹²¹Sb | Stable isotope with natural abundance of about 57.21%. |
| ¹²³Sb | Stable isotope, comprising about 42.79% of natural antimony. |
| ¹²²Sb | Radioactive isotope with a half-life of 2.7238 days. Used in medical research. |
| ¹²⁴Sb | Radioactive isotope with a half-life of 60.20 days. Used in industrial radiography. |
| ¹²⁵Sb | Radioactive isotope with applications in nuclear medicine. |
| ¹²⁶Sb | Radioactive isotope with a relatively short half-life, used in scientific studies. |
The properties and stability of these isotopes make antimony a versatile element in various scientific and industrial applications.
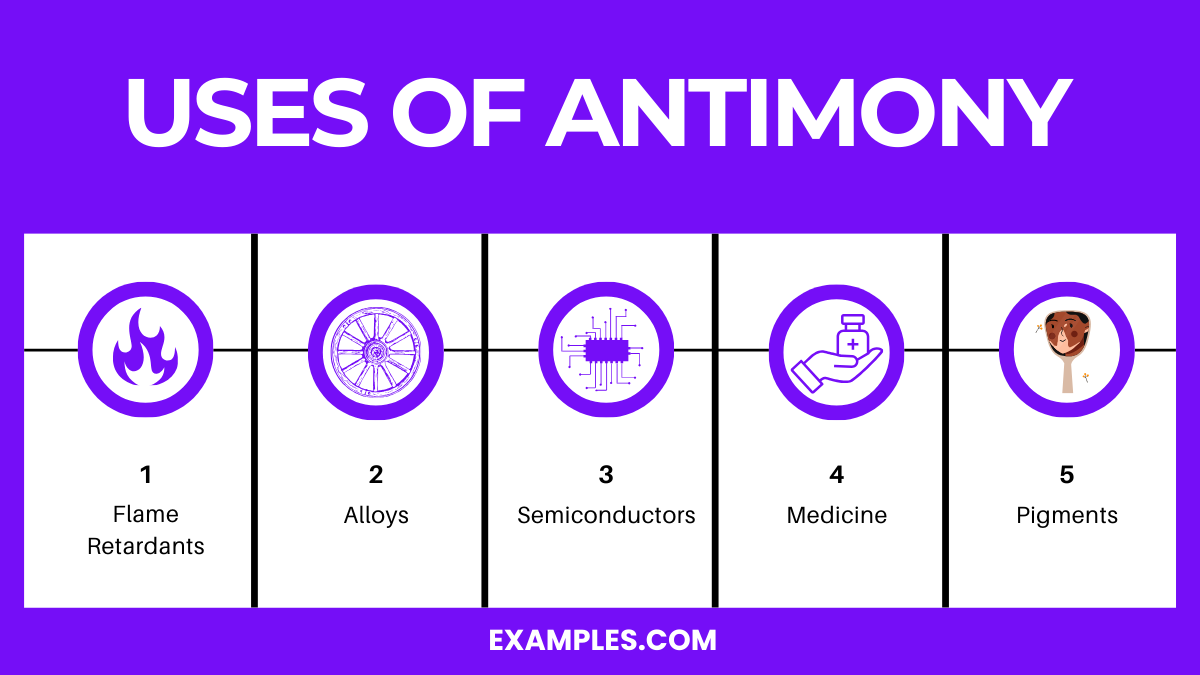
Antimony, a metalloid with the symbol Sb, has several significant uses in various industries due to its unique physical and chemical properties.
One of the primary uses of antimony is as a flame retardant in household goods and consumer electronics. When combined with halogenated compounds, antimony trioxide acts as an effective synergist that enhances the flame-retardant properties of these compounds. It’s used in plastics, textiles, rubber, and paints to prevent the spread of fire.
Antimony is widely used in alloying to increase hardness and mechanical strength. Its most notable application is in lead-acid batteries. The addition of antimony to lead improves the battery’s charge retention and decreases the amount of maintenance required. Antimony alloys are also used in bearings, solder, and pewter.
In the field of semiconductors, antimony is valued for its ability to provide n-type conductivity. Antimony compounds, particularly antimony telluride, are used in thermoelectric devices for converting waste heat into electricity, offering a sustainable way to generate power.
Antimony compounds have historical significance in medicine. Antimony potassium tartrate, once used as an emetic, highlights antimony’s limited but notable role in pharmaceuticals. Modern research is exploring antimony’s potential in treating parasitic diseases like leishmaniasis.
Antimony salts are used to create pigments for paints, plastics, and ceramics. The most common pigment made from antimony is antimony white (antimony oxide), which provides a white coloration and opacity to products.
The commercial production of antimony involves several key steps. The majority of antimony is extracted from its ores, mainly stibnite (Sb₂S₃), which contains up to 71% antimony.
Throughout this process, environmental and safety considerations are crucial due to the toxic nature of some antimony compounds. The production facilities are designed to minimize any adverse environmental impact and ensure the safety of workers handling these compounds.
Antimony, a metalloid, can have various health effects depending on the exposure level and form of antimony. Here are some key points:
Antimony’s environmental impact is significant, especially near mining and processing sites. Here’s an overview:
Antimony is primarily used in alloys, flame retardants, plastics, and electronics, enhancing durability and fire resistance.
Excessive exposure to antimony can be toxic, potentially causing respiratory, skin, and cardiovascular issues.
Common products containing antimony include batteries, flame-resistant materials, ceramics, glass, and some cosmetics.
The old name for antimony is “Stibium,” symbolized as ‘Sb’ in the periodic table.
Touching antimony typically poses minimal risk, but prolonged contact can cause skin irritation.
Antimony is mainly sourced from stibnite ore and as a byproduct of lead and copper smelting.
Antimony’s unique properties make it invaluable in numerous applications, from fire retardants to alloys. However, handling it requires caution due to its potential toxicity. Balancing its industrial benefits with health and environmental considerations is essential for its sustainable and safe use.

Dive into the intriguing world of Antimony with our comprehensive guide, crafted especially for educators. Antimony, a lesser-known yet significant element, holds a unique place in both science and history. As a teacher, understanding its complexities and conveying them to students can be challenging. Our guide simplifies this task, offering clear explanations, practical examples, and engaging teaching tips. Enhance your lessons and captivate your students with our in-depth exploration of Antimony.
Antimony is a chemical element with the symbol Sb (from Latin: stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient times and were used for cosmetics; metallic antimony was also known, but it was erroneously identified as lead upon its discovery. The largest applications for metallic antimony are as alloying material for lead and tin and for lead antimony plates in lead-acid batteries. Alloying lead and tin with antimony improves the properties of the alloys which are used in solders, bullets, and plain bearings.

Formula: Sb
Composition: Consists of a single antimony atom.
Bond Type: Antimony typically forms covalent bonds, leveraging its five valence electrons for bonding.
Molecular Structure: Appears as a lustrous gray metalloid in its solid state.
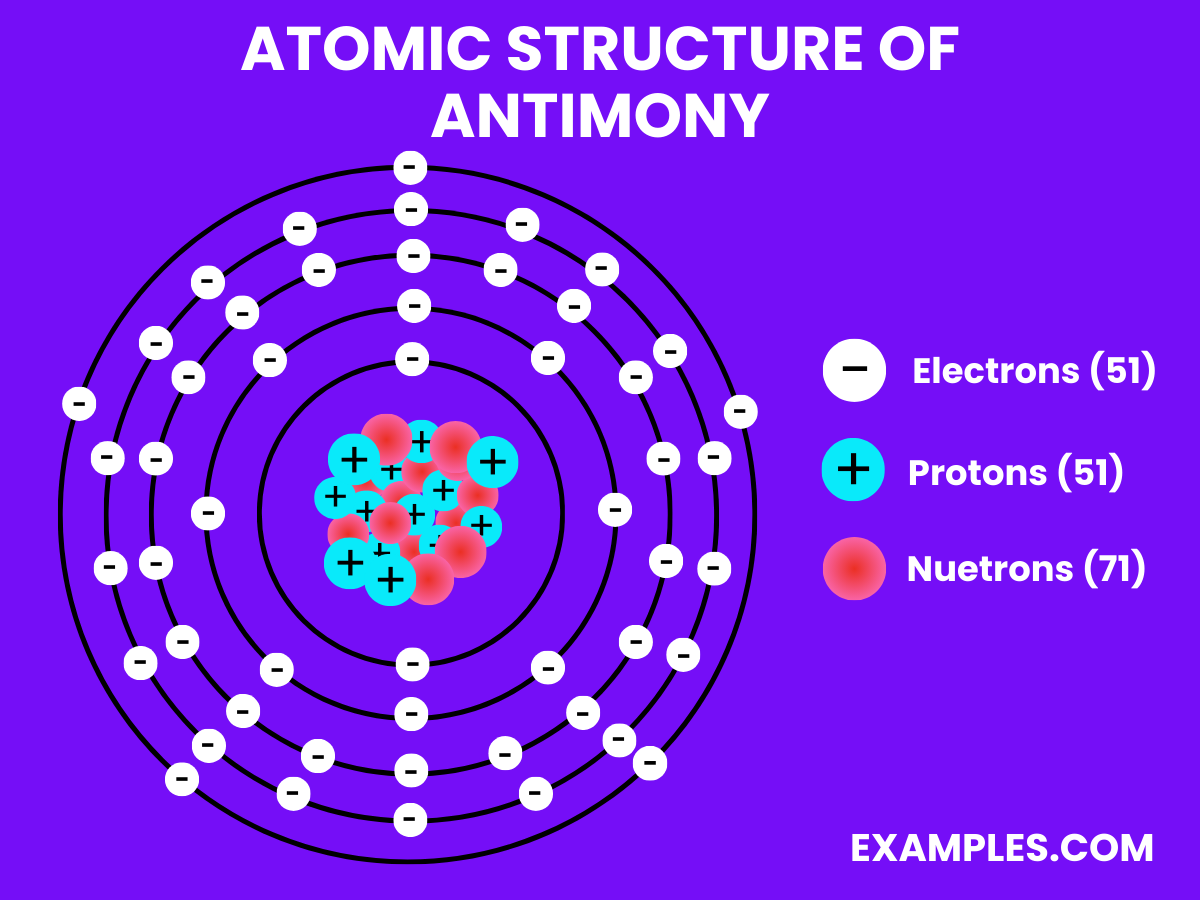
Electron Configuration: Possesses 51 electrons, with the configuration [Kr] 4d¹⁰ 5s² 5p³.
Significance: Widely used in flame retardants, lead-acid batteries, alloys, and in the manufacture of certain microelectronics.
Role in Chemistry: Antimony plays a crucial role in the development of materials science, particularly in alloys and flame retardant compounds.


Property | Description |
|---|---|
Appearance | Silvery, lustrous gray metalloid, exhibiting a metallic shine. |
Density | Approximately 6.697 g/cm³, indicating a relatively high density. |
Melting Point | Melts at about 630.63 °C, showing moderate thermal stability. |
Boiling Point | Boils at 1587 °C, indicative of its high thermal endurance. |
Crystal Structure | Exhibits a rhombohedral crystal structure, contributing to its brittleness. |
Hardness | Fairly hard, though brittle, making it difficult to machine or work with. |
Conductivity | Poor conductor of heat and electricity, useful in certain alloys and compounds. |
State at Room Temperature | Solid under standard conditions, maintaining its structure without degradation. |
Antimony, with the chemical symbol Sb, exhibits several interesting chemical properties:
Reactivity with Oxygen: Antimony reacts with oxygen to form antimony trioxide (Sb₂O₃): 4Sb+3O₂→2Sb₂O₃ This reaction is fundamental in the production of flame retardant materials.
Formation of Stibine: It reacts with hydrogen, forming stibine (SbH₃): Sb+3H₂→2SbH₃ Stibine is a highly toxic gas, similar to arsine.
Reaction with Halogens: Antimony reacts with halogens like chlorine to form antimony trichloride (SbCl₃) and antimony pentachloride (SbCl₅): Sb+3Cl₂→2SbCl₃ Sb+5Cl₂→2SbCl₃ These are important in the synthesis of organometallic compounds.
Formation of Antimonides: With metals, antimony forms antimonides, which are important in semiconductor technology.
Stability in Air: Antimony is relatively stable in air, not reacting significantly at room temperature.
Combination with Sulfur: It forms various sulfides like antimony trisulfide (Sb₂S₃), used in pyrotechnics and matches.
Reaction with Alkalis: Antimony reacts with alkali metals to form antimonates, used in glasses and ceramics.
Property | Description / Value |
|---|---|
Melting Point | 630.63°C (1167.13°F) |
Boiling Point | 1587°C (2889°F) |
Thermal Conductivity | 24.4 W/(m·K) |
Specific Heat | 0.207 J/(g·K) at 25°C |
Heat of Vaporization | 195.2 kJ/mol |
Heat of Fusion | 19.79 kJ/mol |
Property | Description / Value |
|---|---|
Phase at STP | Solid |
Density | 6.697 g/cm³ at 20°C |
Young’s Modulus | 55 GPa |
Tensile Strength | 170 MPa |
Mohs Hardness | 3 |
Elastic Modulus | 55 GPa |
Property | Description / Value |
|---|---|
Magnetic Susceptibility | Diamagnetic |
Electrical Conductivity | 2.5×10^4 S/m at 20°C |
Property | Description / Value |
|---|---|
Atomic Number | 51 |
Atomic Mass | 121.760 u |
Neutron Cross Section | 5.6 barns (for ^121Sb) |
Isotopes | ^121Sb (57.21%), ^123Sb (42.79%) |
Radioactivity | Natural antimony is stable. ^125Sb and ^124Sb are notable radioactive isotopes with half-lives of 2.75856 years and 60.20 days, respectively |
Antimony forms several important compounds, each with unique properties and applications. Here are the top six antimony compounds along with their relevant chemical equations:
Antimony Trichloride (SbCl₃): 2 Sb+3 Cl₂→2 SbCl₃.
Used in flame retardants and as a chemical reagent.
Antimony Trioxide (Sb₂O₃): 4 Sb+3 O₂→2 Sb₂O₃.
Commonly used in flame retardant formulations.
Antimony Pentoxide (Sb₂O₅): Sb₂O₃+O₂→Sb₂O₅.
Utilized as a flame retardant and in some glass and ceramic products.
Antimony Sulfide (Sb₂S₃): 2 Sb+3 S→Sb₂S₃.
Used in pyrotechnics, match heads, and as a pigment.
Sodium Antimonate (NaSbO₃): Formed by reacting antimony trioxide with sodium hydroxide.
Used in glass decolorization and as a catalyst.
Antimony Pentafluoride (SbF₅): 2 Sb+5 F₂→2 SbF₅.
Employed in some chemical synthesis processes as a strong fluorinating agent.
Isotope | Key Characteristics |
|---|---|
¹²¹Sb | Stable isotope with natural abundance of about 57.21%. |
¹²³Sb | Stable isotope, comprising about 42.79% of natural antimony. |
¹²²Sb | Radioactive isotope with a half-life of 2.7238 days. Used in medical research. |
¹²⁴Sb | Radioactive isotope with a half-life of 60.20 days. Used in industrial radiography. |
¹²⁵Sb | Radioactive isotope with applications in nuclear medicine. |
¹²⁶Sb | Radioactive isotope with a relatively short half-life, used in scientific studies. |
The properties and stability of these isotopes make antimony a versatile element in various scientific and industrial applications.

Antimony, a metalloid with the symbol Sb, has several significant uses in various industries due to its unique physical and chemical properties.
One of the primary uses of antimony is as a flame retardant in household goods and consumer electronics. When combined with halogenated compounds, antimony trioxide acts as an effective synergist that enhances the flame-retardant properties of these compounds. It’s used in plastics, textiles, rubber, and paints to prevent the spread of fire.
Antimony is widely used in alloying to increase hardness and mechanical strength. Its most notable application is in lead-acid batteries. The addition of antimony to lead improves the battery’s charge retention and decreases the amount of maintenance required. Antimony alloys are also used in bearings, solder, and pewter.
In the field of semiconductors, antimony is valued for its ability to provide n-type conductivity. Antimony compounds, particularly antimony telluride, are used in thermoelectric devices for converting waste heat into electricity, offering a sustainable way to generate power.
Antimony compounds have historical significance in medicine. Antimony potassium tartrate, once used as an emetic, highlights antimony’s limited but notable role in pharmaceuticals. Modern research is exploring antimony’s potential in treating parasitic diseases like leishmaniasis.
Antimony salts are used to create pigments for paints, plastics, and ceramics. The most common pigment made from antimony is antimony white (antimony oxide), which provides a white coloration and opacity to products.
The commercial production of antimony involves several key steps. The majority of antimony is extracted from its ores, mainly stibnite (Sb₂S₃), which contains up to 71% antimony.
Mining and Extraction: The first step involves mining stibnite ore, which is then subjected to a flotation process. This process separates the antimony sulfide from the waste rock or impurities.
Roasting: The antimony sulfide is roasted in air to convert it into antimony oxide (Sb₂O₃).
Reduction: The antimony oxide is then reduced to metallic antimony by heating it with carbon or by reacting it with iron scrap in a blast furnace.
Refining: The crude antimony is further refined to remove impurities. This is typically done through electrolytic refining or by treating the antimony with oxygen-rich air to remove remaining sulfur and lead.
Formation of Antimony Products: Finally, the refined antimony is processed into alloys, compounds, and various products depending on its intended use.
Throughout this process, environmental and safety considerations are crucial due to the toxic nature of some antimony compounds. The production facilities are designed to minimize any adverse environmental impact and ensure the safety of workers handling these compounds.
Antimony, a metalloid, can have various health effects depending on the exposure level and form of antimony. Here are some key points:
Respiratory Issues: Inhalation of antimony dust or fumes, often in industrial settings, can lead to respiratory irritation, chronic bronchitis, and even pneumoconiosis (a lung disease caused by inhaling dust).
Skin Contact: Direct skin contact with antimony compounds can cause dermatitis and skin irritation. In more severe cases, it can lead to ulcers.
Gastrointestinal Problems: Ingesting antimony compounds can lead to gastrointestinal issues such as nausea, vomiting, and abdominal pain.
Cardiovascular Effects: Long-term exposure to antimony has been linked to increased risks of cardiovascular problems, including alterations in heart rhythm.
Reproductive Health: There is evidence suggesting that antimony exposure can adversely affect reproductive health, potentially causing menstrual irregularities and miscarriages.
Cancer Risk: The International Agency for Research on Cancer (IARC) has classified antimony trioxide as a possible human carcinogen, indicating a potential risk of cancer with prolonged exposure.
Neurological Effects: Chronic exposure to antimony can lead to headaches, insomnia, and in some cases, dizziness and depression.
Immunological Impact: There is some indication that antimony exposure can affect the immune system, though the extent and nature of this impact require further research.
Antimony’s environmental impact is significant, especially near mining and processing sites. Here’s an overview:
Water Contamination: Antimony can leach into water bodies from industrial processes, leading to contamination. This is particularly concerning for aquatic life.
Soil Contamination: Antimony compounds can accumulate in soils, especially near industrial areas, affecting soil quality and potentially entering the food chain.
Air Pollution: Antimony trioxide and other compounds, when airborne, contribute to air pollution. This is a concern for both environmental and human health.
Impact on Aquatic Life: Antimony in water bodies can be toxic to fish and other aquatic organisms, affecting their growth, reproduction, and survival.
Bioaccumulation: Antimony can bioaccumulate in plants and animals, leading to higher concentrations in organisms higher up in the food chain.
Effect on Plant Growth: High concentrations of antimony in soil can inhibit plant growth and can cause phytotoxicity.
Long-Term Persistence: Antimony compounds can persist in the environment for long periods, leading to prolonged exposure risks for both wildlife and humans.
Waste Management Challenges: The disposal of antimony-containing waste requires careful handling due to its toxic nature, posing challenges in waste management and recycling processes.
Antimony is primarily used in alloys, flame retardants, plastics, and electronics, enhancing durability and fire resistance.
Excessive exposure to antimony can be toxic, potentially causing respiratory, skin, and cardiovascular issues.
Common products containing antimony include batteries, flame-resistant materials, ceramics, glass, and some cosmetics.
The old name for antimony is “Stibium,” symbolized as ‘Sb’ in the periodic table.
Touching antimony typically poses minimal risk, but prolonged contact can cause skin irritation.
Antimony is mainly sourced from stibnite ore and as a byproduct of lead and copper smelting.
Antimony’s unique properties make it invaluable in numerous applications, from fire retardants to alloys. However, handling it requires caution due to its potential toxicity. Balancing its industrial benefits with health and environmental considerations is essential for its sustainable and safe use.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Antimony?
49
51
53
55
Antimony is primarily used in the manufacturing of:
Paints
Pesticides
Fire retardants
Pharmaceuticals
Which mineral is the most common source of antimony?
Galena
Hematite
Stibnite
Bauxite
Antimony is classified chemically as a:
Metal
Metalloid
Non-metal
Gas
Which of the following statements about antimony's reactivity is true?
It is highly reactive with water.
It does not react with acids.
It reacts slowly with oxygen at room temperature.
It is inert to chemical reactions.
In which period of the periodic table is antimony found?
4th
5th
6th
7th
Antimony's alloys are primarily used for:
Electrical insulation
Increase fluidity in metals
Hardening of lead
Producing semi-conductors
Acute exposure to antimony can cause:
Skin elasticity improvement
Respiratory issues
Enhanced vision
Digestive stability
The discovery of antimony was first documented by:
Alchemists in the Middle Ages
Ancient Egyptians
Roman scholars
Greek philosophers
Antimony is often found combined with:
Carbon
Sulfur
Silicon
Hydrogen
Before you leave, take our quick quiz to enhance your learning!

