What is the atomic number of Argon?
16
18
20
22
Embark on an educational journey into the inert world of Argon, a noble gas that’s more than just filler in our atmosphere. This 75-word guide is packed with engaging examples, practical applications, and essential information, making it the perfect tool for teachers looking to enrich their science curriculum. From shielding our welds to lighting our streets, Argon’s versatility is as expansive as the universe. Equip yourself with the knowledge to make Argon a standout topic in your classroom.
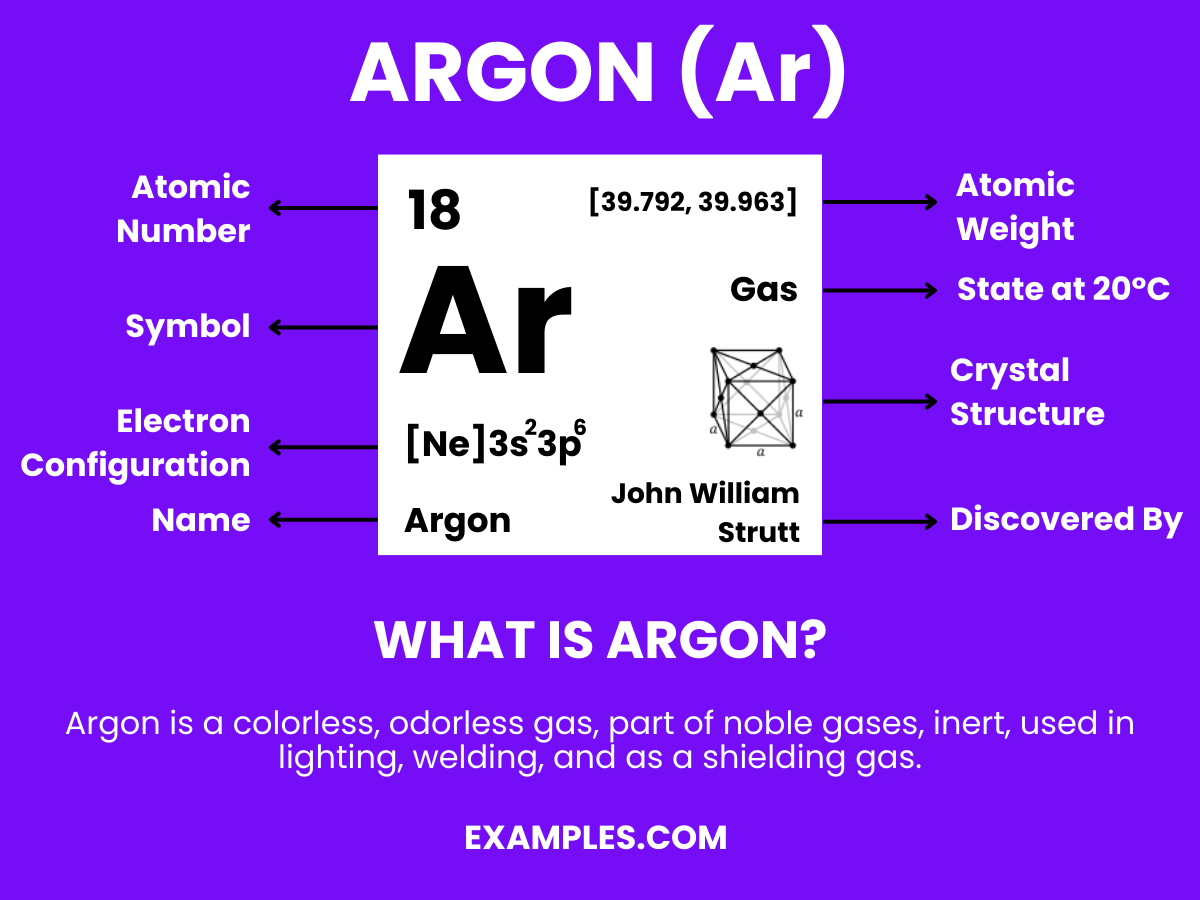
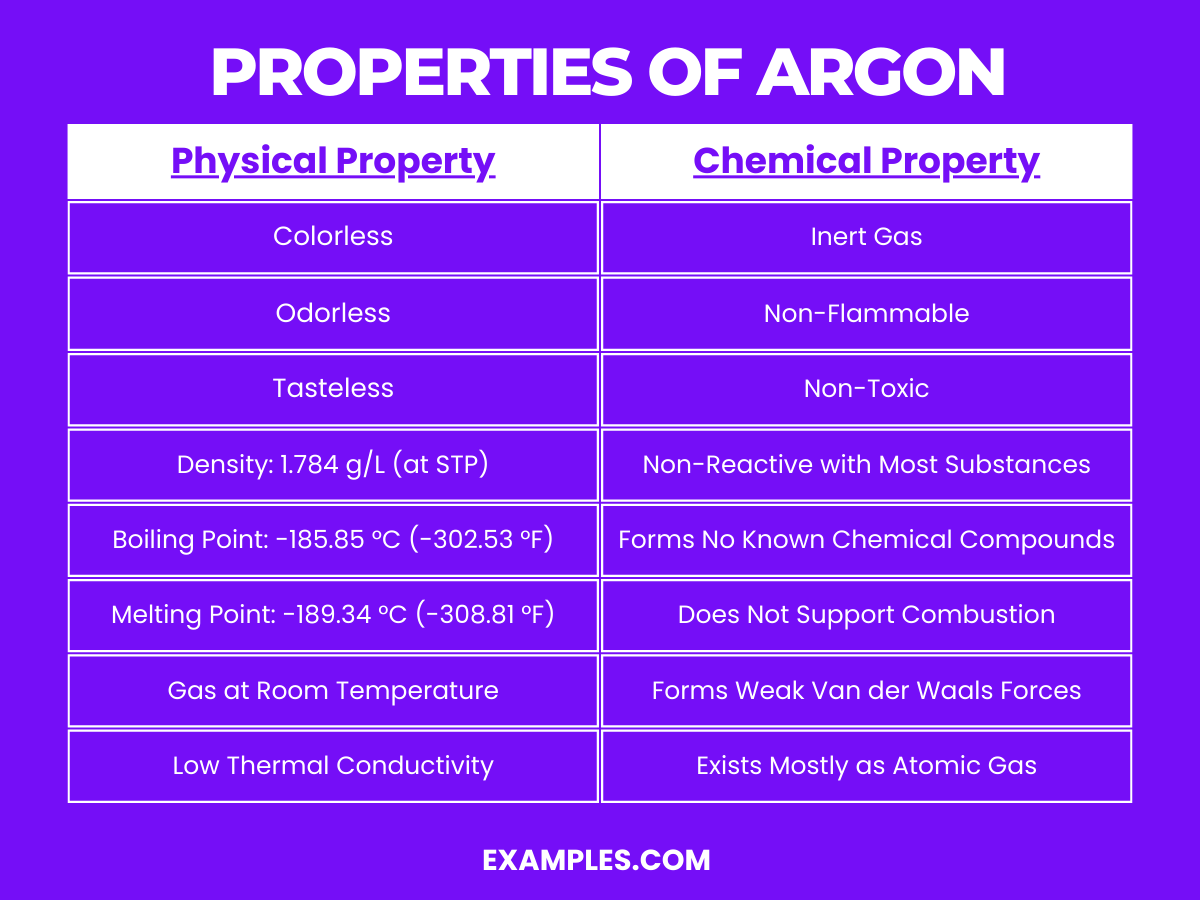
Argon is a colorless, odorless, and tasteless noble gas with the chemical symbol Ar and atomic number 18. It’s the third most abundant gas in the Earth’s atmosphere and is known for its inertness, rarely participating in chemical reactions. Argon’s lack of reactivity makes it highly valuable in applications that require a non-reactive atmosphere, from preserving historical documents to protecting welds in industrial processes. Its role in fluorescent lighting and other technologies makes it an everyday invisible hero.
| Helium |
| Neon |
| Krypton |
| Xenon |
| Radon |
| Property | Description |
|---|---|
| State at Room Temperature | Gas |
| Color | Colorless |
| Odor | Odorless |
| Taste | Tasteless |
| Density | 1.784 g/L at 0°C and 1 atm |
| Boiling Point | -185.85 °C (-302.53 °F) |
| Melting Point | -189.34 °C (-308.81 °F) |
| Thermal Conductivity | Low (17.72 mW/m·K at 300K) |
Argon is a noble gas and exhibits typical characteristics of its group. Its chemical properties are defined by its inertness:
| Property | Description / Value |
|---|---|
| Melting Point | -189.34°C (-308.81°F) |
| Boiling Point | -185.848°C (-302.526°F) |
| Thermal Conductivity | 0.01772 W/(m·K) at 300K |
| Specific Heat | 0.5203 J/(g·K) at 20°C |
| Heat of Vaporization | 6.53 kJ/mol |
| Heat of Fusion | 1.18 kJ/mol |
| Critical Temperature | -122.4°C (-188.3°F) |
| Property | Description / Value |
|---|---|
| Phase at STP | Gas |
| Density (at STP) | 1.784 g/L |
| Solubility in Water | 33.6 cm³/kg at 20°C |
| Color | Colorless |
| Odor | Odorless |
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | -19.6·10⁻⁶ cm³/mol (Diamagnetic) |
| Electrical Conductivity | Non-conductor (as a noble gas) |
| Property | Description / Value |
|---|---|
| Atomic Number | 18 |
| Atomic Mass | 39.948 u |
| Neutron Cross Section | 0.675 barns (for ^40Ar) |
| Isotopes | ^36Ar (0.334%), ^38Ar (0.063%), ^40Ar (99.604%) |
| Radioactivity | ^37Ar, ^39Ar, ^42Ar are radioactive isotopes with half-lives ranging from 35 days to 32.9 years |
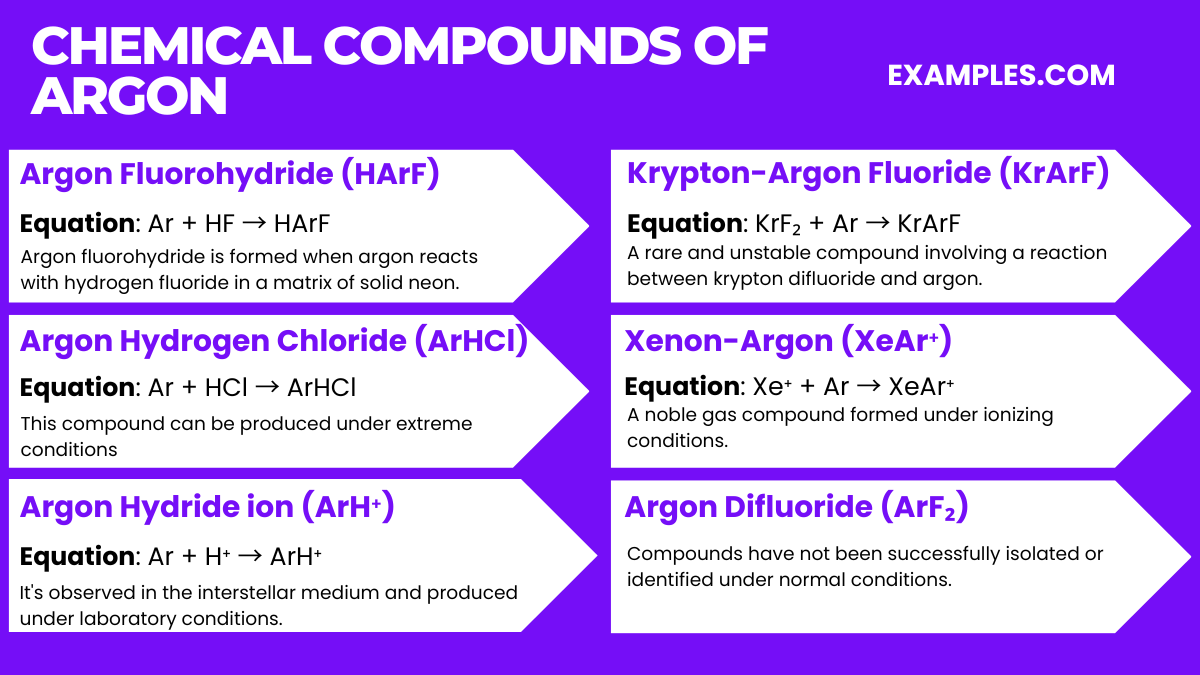
Argon is a noble gas and is known for its lack of reactivity, thus it has very few compounds, and those that do exist are typically only stable under extreme conditions. Here are some of the most notable argon compounds:
| Isotope | Abundance | Atomic Mass | Half-Life | Description |
|---|---|---|---|---|
| Argon-36 | ~0.34% | 35.9675 u | Stable | Found in the atmosphere, and used in dating water and ice samples. |
| Argon-38 | ~0.06% | 37.9627 u | Stable | Least abundant stable isotope, occurring in the Earth’s atmosphere. |
| Argon-39 | Trace (radioactive) | 39.9624 u | 269 years | Produced by cosmic ray activity, used in argon-argon dating similar to carbon dating. |
| Argon-40 | ~99.6% | 39.9624 u | Stable | The most abundant isotope, resulting from the decay of Potassium-40, used in K-Ar dating. |
| Argon-41 | Trace (radioactive) | 40.9645 u | 1.83 hours | Formed by the interaction of atmospheric argon with cosmic rays, decays back to stable Potassium-41. |
| Argon-42 | Synthetic (radioactive) | 41.9630 u | 32.9 years | Produced in nuclear reactors, it is not naturally occurring due to its rapid decay and low production in nature. |
The isotopes of argon provide valuable information and applications in various scientific fields, from environmental studies to archeological dating. The stable isotopes (Argon-36, Argon-38, and Argon-40) are naturally occurring and found in the atmosphere, whereas the others are primarily of academic interest or are used in specific scientific applications.
Argon is most commonly produced as a byproduct of oxygen and nitrogen production through the fractional distillation of liquid air, a process in which air is cooled to a liquid state and then gradually heated in a distillation column. Here’s a detailed look at the process:
Argon is a noble gas that is colorless, odorless, tasteless, and non-toxic in its natural state. It is generally considered to be inert and has minimal chemical reactivity. However, like any substance, it can have health effects under certain conditions:
In general, standard safety practices in industries that use argon (like ensuring proper ventilation and using appropriate protective equipment) are effective in preventing health issues.
Argon is the third most abundant gas in the Earth’s atmosphere, making up about 0.934% by volume. It is a naturally occurring element that plays a specific role in the environment:
Argon’s health effects are primarily related to its physical properties, such as its ability to displace oxygen, rather than its chemical properties, which are minimal due to its inertness. Environmentally, argon is a benign gas that has no significant ecological impact or environmental hazard due to its stability and inert nature. Its effects are largely related to its use and handling by humans rather than its presence or activity in the natural environment.
Argon is primarily used in welding, lighting, insulating windows, and preserving historical documents due to its inert nature.
Argon is non-toxic but can displace oxygen, leading to asphyxiation in poorly ventilated spaces.
Argon is found in the Earth’s atmosphere, constituting about 0.93% of it, and is obtained from air separation.
Argon is unreactive due to its full outer electron shell, making it stable and inert under most conditions.
The density of argon at standard temperature and pressure is 1.784 grams per liter.
Argon is a versatile, inert gas with varied applications, from welding to preserving artifacts. Its non-reactivity and safety make it valuable in industrial and scientific settings. Understanding its properties, uses, and safe handling is key for leveraging Argon effectively. Stay informed and cautious to harness Argon’s benefits while mitigating risks in your projects or studies.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
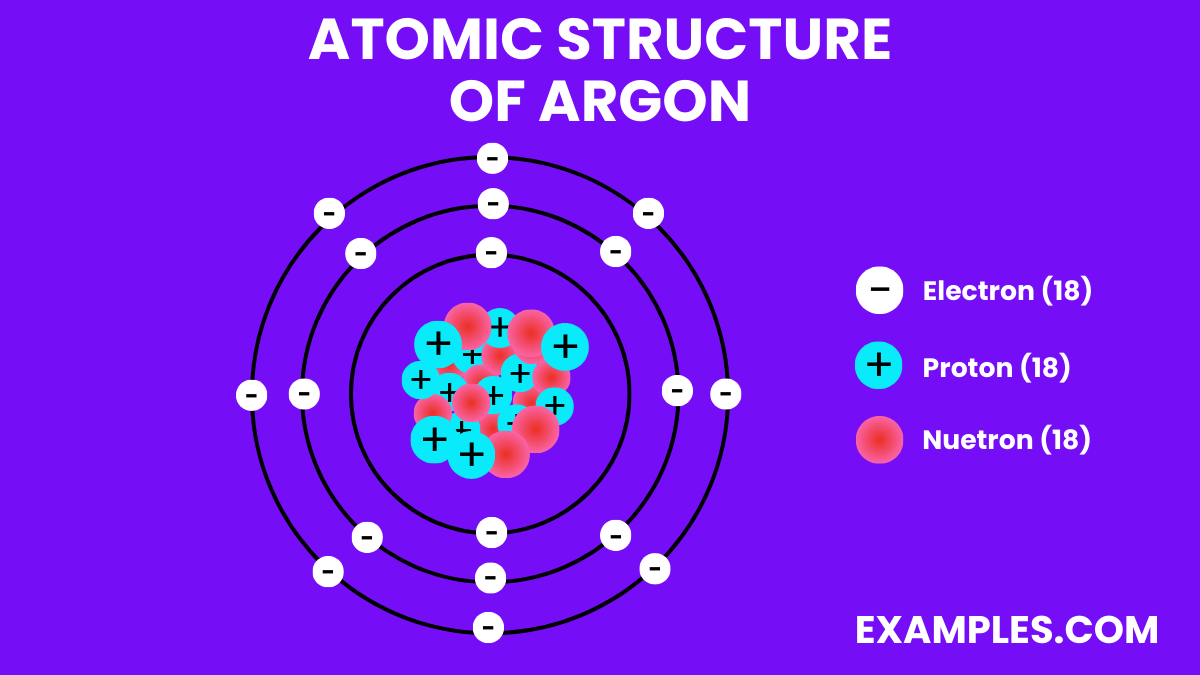
Electrons
Neutrons
Protons
What is the atomic number of Argon?
16
18
20
22
Argon is primarily used in:
Welding
Water treatment
Refrigeration
Battery production
Which of the following is a characteristic of Argon?
Highly reactive
Colorless
Smells strongly
Conducts electricity
Argon belongs to which group on the periodic table?
Alkali metals
Transition metals
Noble gases
Halogens
What percentage of the Earth's atmosphere is composed of Argon?
About 0.93%
About 1.28%
About 20.95%
About 78.09%
The discovery of Argon was made by:
Henry Cavendish
John Dalton
William Ramsay
Antoine Lavoisier
Argon is used in incandescent light bulbs to:
Increase brightness
Prevent oxygen from corroding the filament
Change the color of the light
Reduce energy consumption
Which isotopic form of Argon is most abundant in nature?
Argon-36
Argon-38
Argon-40
Argon-42
How does Argon remain stable?
By losing electrons
By gaining electrons
By sharing electrons
It already has a full outer electron shell
In medical applications, Argon is used for:
Sterilization
Anesthesia
Cryosurgery
Blood pressure monitoring
Before you leave, take our quick quiz to enhance your learning!

