What is the atomic number of Arsenic?
33
32
74
51
Dive into the world of Arsenic with our detailed guide, tailored for educators. Arsenic, often misunderstood, plays a crucial role in various fields. This guide not only clarifies its definition and meaning but also offers practical usage tips. By incorporating real-world examples, we make understanding Arsenic straightforward and relevant. Ideal for teachers, this resource simplifies complex concepts, ensuring you can convey its significance effectively to your students.
Arsenic is a naturally occurring chemical element, known for its paradoxical presence in nature. It’s found both as a life-sustaining trace element and a potentially harmful contaminant. This dual nature makes understanding Arsenic essential, especially in fields like environmental science and chemistry. Simple yet comprehensive, our definition helps teachers explain Arsenic’s complexity to students, fostering a deeper understanding of its impact on the world around us.
| Boron (B) |
| Silicon (Si) |
| Germanium (Ge) |
| Antimony (Sb) |
| Tellurium (Te) |
| Property | Detail |
|---|---|
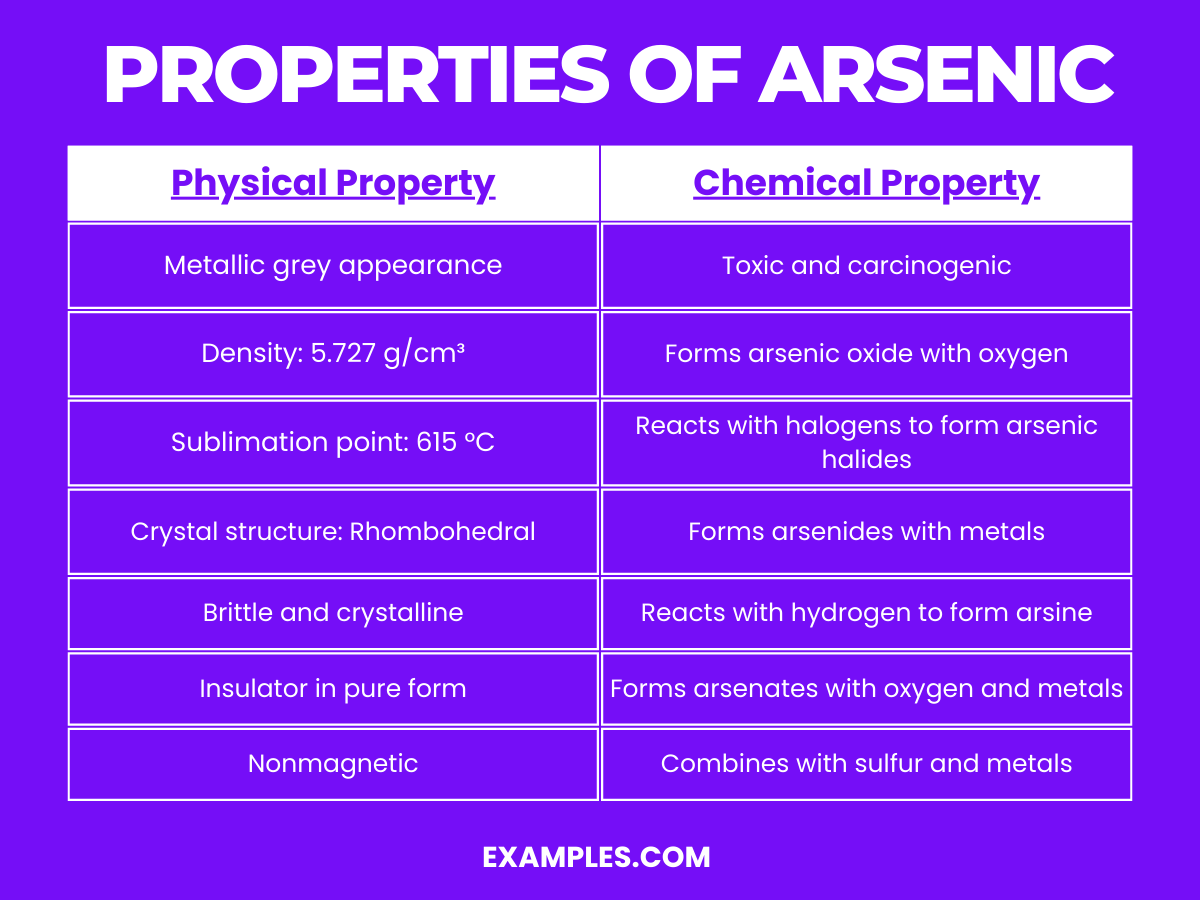
| Appearance | Metallic grey, crystalline solid |
| Density | 5.727 g/cm³ at 20°C, one of the densest elements |
| Melting Point | Sublimes at 615°C, does not have a liquid state at atmospheric pressure |
| Boiling Point | 613°C (sublimation point) |
| Crystal Structure | Rhombohedral, similar to the crystal structure of antimony |
| State at Room Temperature | Solid, but it sublimates (directly transitions from solid to gas) at higher temperatures |
| Hardness | Brittle, easily fractured |
| Electrical Conductivity | Poor conductor in its grey form, semi-metallic properties |
Arsenic, a metalloid, exhibits a range of chemical properties due to its position in the periodic table.
| Property | Value with Unit |
|---|---|
| Boiling Point | 615 °C (Sublimation) |
| Melting Point | 817 °C (under pressure) |
| Critical Temperature | Not applicable |
| Critical Pressure | Not applicable |
| Heat of Vaporization | (Sublimation) 34.76 kJ/mol |
| Heat of Fusion | 24.44 kJ/mol (at 817 °C) |
| Specific Heat Capacity (at 25°C) | 0.328 J/g·K |
| Thermal Conductivity | 50.2 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at 20°C) | 5.727 g/cm³ |
| Mohs Hardness | 3.5 |
| Solubility | Insoluble in water |
| Appearance | Metallic grey |
| Crystal Structure | Rhombohedral |
| Phase at Room Temperature | Solid |
| Property | Value with Unit |
|---|---|
| Electrical Resistivity (at 20°C) | 333 µΩ·m |
| Thermal Conductivity | 50.2 W/m·K |
| Electronegativity (Pauling scale) | 2.18 |
| Ionization Energy | First: 9.7886 eV |
| Property | Value with Unit |
|---|---|
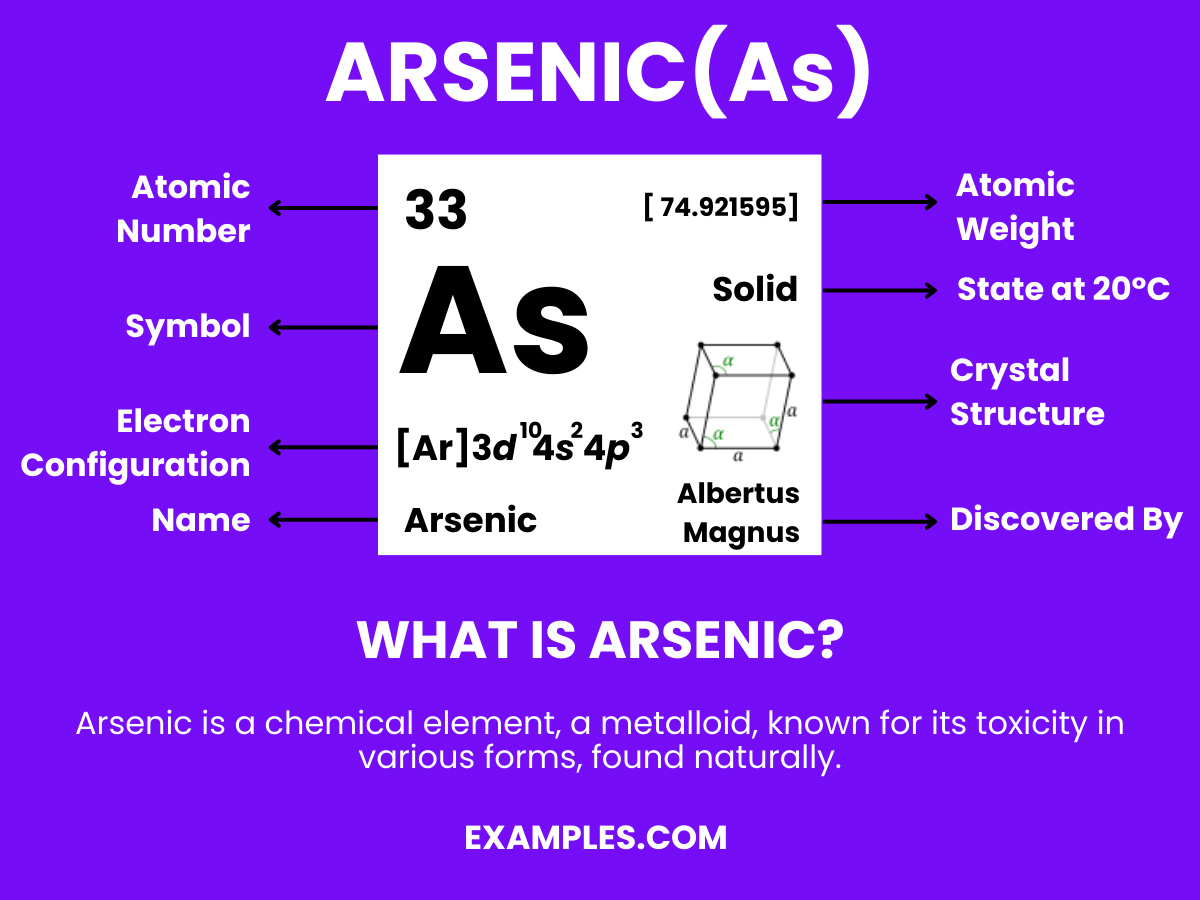
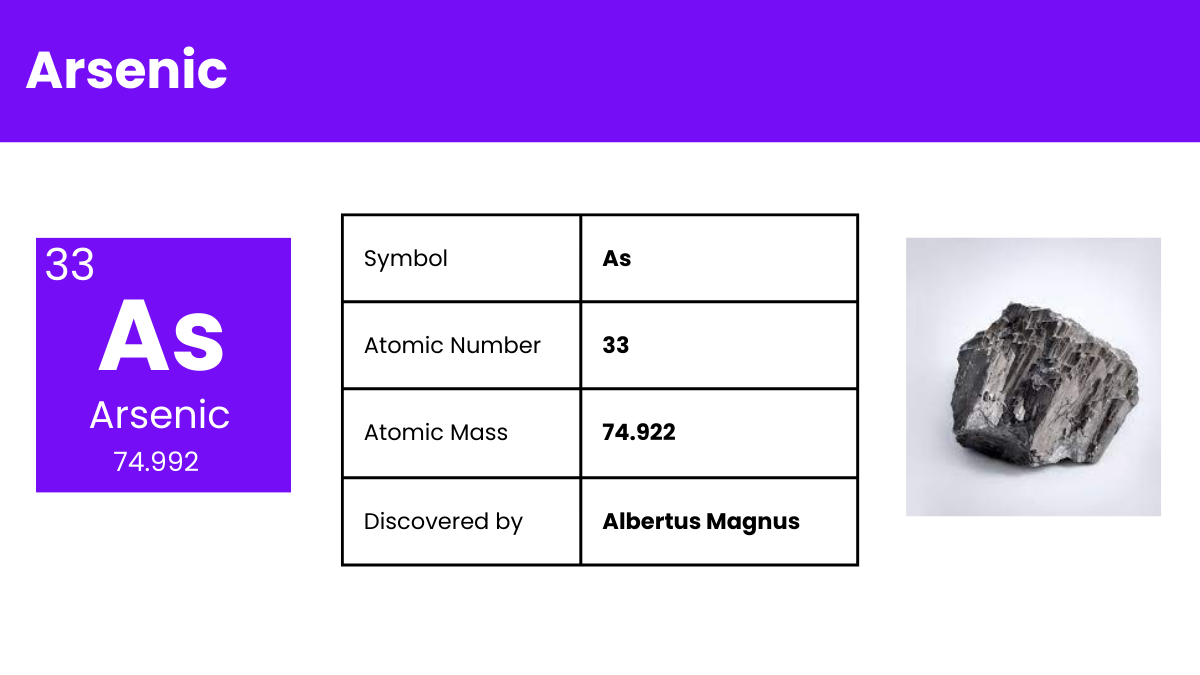
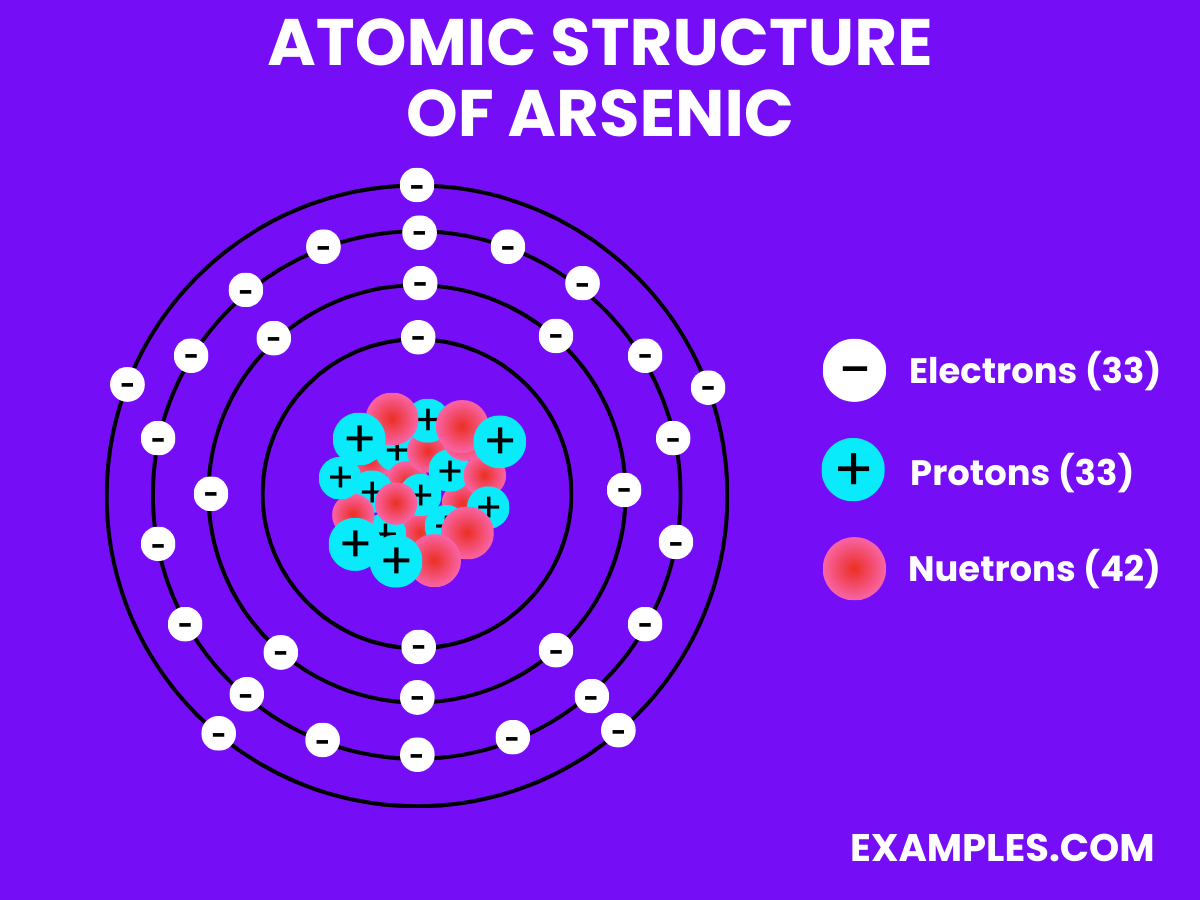
| Atomic Number | 33 |
| Atomic Mass | 74.921595 amu |
| Isotopes | ^75As (100%) |
| Nuclear Spin (for ^75As) | 3/2 ℏ |
| Neutron Cross Section (for ^75As) | 4.5 barns |
| Nuclear Magnetic Moment (for ^75As) | 1.4398 µN |
Arsenic forms several important chemical compounds, each with distinct properties and applications. Here are the top six arsenic compounds:
| Isotope | Atomic Mass | Half-Life | Notes |
|---|---|---|---|
| ⁷⁴As | 73.9239 | 17.77 days | Used in nuclear medicine and research. |
| ⁷⁵As | 74.9216 | Stable | The only stable isotope, common in nature. |
| ⁷⁶As | 75.9214 | 26.3 hours | Beta emitter, used in biochemical research. |
| ⁷⁷As | 76.9206 | 38.83 hours | Used in tracer studies in environmental research. |
| ⁷⁸As | 77.9218 | 90.7 minutes | Decays to stable ⁷⁸Se, used in nuclear medicine. |
| ⁷⁹As | 78.9208 | 9.01 days | Radioactive, used in scientific studies. |
Arsenic has been historically used in pesticides and herbicides due to its toxic properties. While the usage has declined due to environmental and health concerns, it remains a component in some wood preservatives and weed killers. These compounds help in controlling pests and unwanted vegetation, ensuring crop protection.
Arsenic compounds, especially chromated copper arsenate (CCA), have been used extensively for wood preservation. They protect wood from rot, decay, and termite infestation, making them ideal for outdoor applications like decks and playground equipment.
In glass manufacturing, arsenic removes impurities, leading to clearer glass products. It acts as a decolorizer, countering the effects of other elements that might cause coloration in the glass.
Arsenic is crucial in the semiconductor industry. When combined with gallium (as gallium arsenide, GaAs), it creates a compound essential for making diodes, transistors, and integrated circuits. These components are vital for the functioning of mobile phones, solar cells, and satellite communications.
Although its use has decreased, arsenic has historical significance in medicine. Arsenic trioxide, specifically, is used in the treatment of a type of cancer known as acute promyelocytic leukemia (APL). It helps induce remission in patients with this specific cancer type.
The commercial production of arsenic primarily involves the processing of arsenic-containing minerals, the most common being arsenopyrite (FeAsS). The production process typically includes the following steps:
This production process must be carefully controlled to manage the toxic nature of arsenic and minimize environmental and health risks associated with arsenic exposure.
Arsenic, a naturally occurring element, can have significant health effects, especially when exposure occurs over a prolonged period or at high levels. The impact on health varies depending on the form and concentration of arsenic, as well as the route and duration of exposure.
In cases of high-level exposure, acute arsenic poisoning can occur. Symptoms include severe gastrointestinal distress, such as vomiting and diarrhea, often accompanied by abdominal pain and cramps. In extreme cases, it can lead to shock, coma, and even death.
Long-term exposure to lower levels of arsenic, often found in contaminated water or food, can lead to chronic arsenic poisoning. This manifests as skin problems (like pigmentation changes and lesions), peripheral neuropathy, gastrointestinal symptoms, and a feeling of general malaise.
Arsenic is a well-known carcinogen. Chronic exposure increases the risk of various cancers, including skin, lung, bladder, and kidney cancers. The risk is heightened in individuals who consume arsenic-contaminated water over extended periods.
Long-term arsenic exposure has been linked to cardiovascular diseases, including hypertension, heart disease, and stroke. It also poses a risk to lung health, contributing to chronic bronchitis and other pulmonary issues.
Pregnant women exposed to arsenic face a higher risk of miscarriages, stillbirths, and preterm births. Additionally, arsenic exposure can affect a child’s cognitive development and increase the risk of illnesses in later life.
Arsenic, while a natural component of the Earth’s crust, can become a significant environmental pollutant due to human activities. Its environmental effects are extensive and concerning.
One of the primary environmental concerns is the contamination of groundwater. Arsenic can leach into water sources from natural deposits or as a result of industrial processes, leading to contamination of drinking water, which poses serious health risks to human and animal populations.
Arsenic can accumulate in soils, especially near mining sites or areas where arsenic-laden pesticides and herbicides have been used. This leads to reduced soil fertility and can result in the accumulation of arsenic in crops, thus entering the food chain.
In aquatic environments, arsenic can be toxic to fish, amphibians, and other aquatic organisms. It can disrupt various physiological processes in marine life, leading to decreased reproduction rates and increased mortality.
Arsenic can bioaccumulate in the tissues of plants and animals, posing a risk to species higher up the food chain, including humans. This accumulation can have long-term ecological impacts.
Arsenic can also be released into the air through industrial processes, such as smelting and coal combustion. This contributes to air pollution and can have adverse effects on respiratory health in humans and animals.
Arsenic exposure can lead to severe health issues, including skin lesions, organ damage, and increased cancer risk.
Arsenic is often found in groundwater, industrial areas, pesticides, and some foods, particularly rice and seafood.
Arsenic in solid form isn’t typically harmful to touch, but its dust or soluble forms can be toxic.
Yes, arsenic is a slow poison, causing gradual health deterioration over long-term exposure, often unnoticed.
Arsenic, a naturally occurring element, has diverse uses but poses significant health risks. It’s crucial in various industries, yet its toxicity necessitates careful handling and awareness. Understanding arsenic’s properties, applications, and health impacts is key for safe usage and environmental preservation.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Arsenic?
33
32
74
51
Arsenic is commonly found in nature combined with:
Iron
Sulfur
Oxygen
All of the above
Which form of arsenic is predominantly toxic?
Organic arsenic
Inorganic arsenic
Elemental arsenic
Gaseous arsenic
Arsenic was historically used in:
Pesticides
Paints
Medicines
All of the above
What is the primary health risk associated with arsenic exposure?
Skin irritation
Lung cancer
Cardiovascular diseases
All of the above
Which test is used to detect arsenic contamination in drinking water?
Marsh test
Flame test
Molybdenum blue test
Silver nitrate test
The removal of arsenic from water is commonly performed by:
Filtration
Distillation
Coagulation
Reverse osmosis
Which industry is a major source of arsenic pollution?
Textile
Semiconductor
Mining
Agriculture
What is a common symptom of acute arsenic poisoning?
Jaundice
Seizures
Hair loss
Vomiting
What role does arsenic play in the semiconductor industry?
It is used in the production of light-emitting diodes (LEDs).
It is used as a dopant in silicon chips.
It is used in the production of gallium arsenide.
It is used as an insulator in microchips.
Before you leave, take our quick quiz to enhance your learning!

