What is the atomic number of Astatine?
83
84
85
86
Dive into the enigmatic world of Astatine, a rare and mysterious element that sits on the edge of scientific exploration. Known for its scarcity and radioactivity, astatine holds a unique place in the periodic table as one of the least understood elements. Our guide unfolds the intriguing aspects of astatine, from its atomic structure and properties to its potential applications and the complex compounds it forms. Join us as we navigate through the fascinating details of astatine, shedding light on its definition, meaning, and the innovative uses that make it a subject of ongoing research and curiosity in the field of chemistry. Discover why astatine continues to captivate scientists and researchers worldwide, and how this elusive element could unlock new frontiers in science and technology.
What is Astatine?
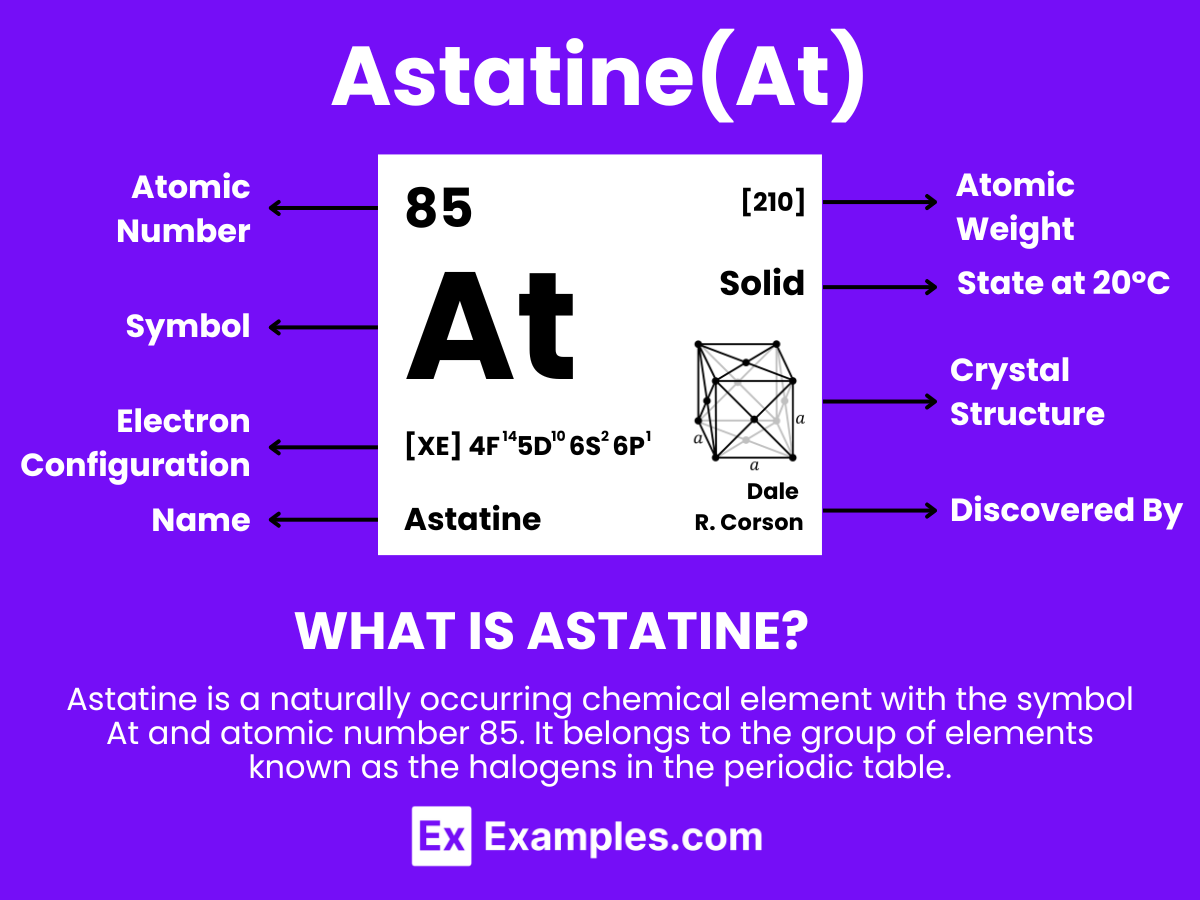
Astatine is a rare and highly radioactive element with the symbol At and atomic number 85. Being the heaviest halogen, astatine was synthesized in 1940 by Dale R. Corson, Kenneth Ross MacKenzie, and Emilio Segrè, who named it after the Greek word for “unstable” (astatos) due to its rarity and radioactivity. It’s found in the Earth’s crust only in trace amounts and is produced synthetically in particle accelerators. Astatine has potential applications in nuclear medicine, particularly in radiotherapy for certain types of cancer. However, its extreme rarity and radioactivity require careful handling and limit its practical applications.
Astatine, unlike its lighter halogen counterparts, is a highly radioactive halogen known for its instability and rarity. This element stands out due to its position in the periodic table, being part of the halogen group but exhibiting some metallic characteristics.
Astatine’s place as a halogen and its unique properties, including its radioactivity and potential in medicine, make it a subject of interest despite its scarcity and the challenges associated with its study and use.
| Property | Description |
|---|---|
| Appearance | Likely to be a metallic; however, the exact appearance is unknown due to its extreme rarity. |
| State at Room Temperature | Solid (presumed due to its position in the periodic table). |
| Melting Point | 302°C (576°F) (estimated). |
| Boiling Point | 337°C (639°F) (estimated). |
| Density | Unknown; predictions vary due to lack of sufficient data. |
| Radioactivity | Highly radioactive, with a half-life of its most stable isotope, Astatine-210, of only 8.1 hours. Emits alpha particles. |
Astatine is a rare and highly radioactive element with the following chemical properties:
| Property | Value |
|---|---|
| Half-Lives of Most Stable Isotopes | Varies (hours to minutes) |
| Primary Decay Modes | Alpha emission, Beta decay |
| Neutron Cross Section | Unknown |
| Isotopic Abundance | Trace amounts |
Astatine, with its elusive nature and rare occurrence, is synthesized through intricate processes. Here are five key points regarding the preparation process of astatine:
Astatine dioxide is a hypothetical compound, showcasing astatine’s potential for different oxidation states, similar to polonium dioxide.
Equation: 2At + O₂ → 2AtO₂
Astatine monoxide represents another oxide of astatine, indicating the element’s capability to exhibit varied oxidation states.
Equation: 2At + O₂ → 2AtO
Astatine hydride, analogous to hydrogen compounds of other halogens, highlights astatine’s interaction with hydrogen.
Equation: At + H₂ → AtH
Astatine tetrachloride exemplifies astatine’s ability to form halides, crucial for synthetic chemistry research and potential applications.
Equation: At + 2Cl₂ → AtCl₄
Astatine oxychloride is a theoretical compound demonstrating astatine’s reactivity with chlorine and oxygen, suggesting potential for diverse chemical behaviors.
Equation: AtCl₄ + H₂O → AtOCl₂ + 2HCl
Astatine hydrate represents a conjectural hydrated form of astatine compounds, indicating the possibility for a wide range of chemical studies.
Equation: AtO₂ + nH₂O → AtO₂·nH₂O
| Isotope | Mass Number | Half-Life | Mode of Decay |
|---|---|---|---|
| At-209 | 209 | 5.41 hours | α decay, β+ decay |
| At-210 | 210 | 8.1 hours | α decay, β+ decay |
| At-211 | 211 | 7.214 hours | α decay (most used for therapy) |
| At-212 | 212 | 0.31 seconds | β− decay, α decay |
| At-213 | 213 | 125 nanoseconds | α decay |
| At-214 | 214 | 558 milliseconds | β− decay, α decay |
| At-215 | 215 | 0.1 milliseconds | α decay |
| At-216 | 216 | 3 seconds | β− decay |
| At-217 | 217 | 32.3 milliseconds | α decay |
| At-218 | 218 | 1.5 seconds | α decay, β− decay |
| At-219 | 219 | 56 seconds | α decay |
| At-220 | 220 | 3.71 minutes | α decay |
| At-221 | 221 | 2.3 minutes | α decay |
| At-222 | 222 | 54 seconds | α decay |
| At-223 | 223 | 50 seconds | α decay |
| At-224 | 224 | 2.5 hours | β− decay |
Antistatic Devices: While polonium is used for removing static electricity, astatine’s extreme rarity and shorter half-life make it impractical for such applications.
Nuclear Batteries: Astatine isotopes, such as astatine-210, could theoretically be used in nuclear batteries by converting the alpha particles they emit into heat, and subsequently into electricity. However, due to its half-life and scarcity, this application is not feasible on a practical scale.
Neutron Sources: Astatine is not known to be used as a neutron source. Unlike polonium, it does not produce neutrons when mixed with beryllium, and its short half-life would make it inefficient for such purposes.
Radioisotope Thermoelectric Generators (RTGs): Due to its short half-life, astatine is not suitable for use in RTGs, which require long-lived isotopes like polonium-210 to provide continuous power over extended periods.
Astatine Periodic: It is a rare element with distinctive properties that allow for potential specialized uses, particularly in the field of nuclear medicine.
Radiation Therapy: Astatine-211 is known for its potential in targeted alpha therapy (TAT) for treating cancer. Its ability to emit alpha particles can destroy malignant cells with minimal impact on surrounding healthy tissue.
Astatine is an element shrouded in mystery due to its rarity and the difficulties involved in studying it. While it shares some properties with polonium, its practical applications are currently limited to scientific research, particularly in the potential treatment of cancer through targeted alpha therapy. Its potential for other uses is hindered by its short half-life and the extreme care required in its handling due to its radioactivity. The exploration of astatine’s properties and uses is an ongoing area of scientific research, and future discoveries may lead to new applications.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Astatine?
83
84
85
86
What is the chemical symbol for Astatine?
As
At
Ac
An
Astatine belongs to which group in the periodic table?
Group 15
Group 16
Group 17
Group 18
What is the most stable isotope of Astatine?
Astatine-209
Astatine-210
Astatine-211
Astatine-212
Astatine is classified as which type of element?
Metal
Metalloid
Nonmetal
Noble gas
In which state is Astatine most likely to be found at room temperature?
Solid
Liquid
Gas
Plasma
What is a key characteristic of Astatine due to its position in the halogen group?
High reactivity
High abundance
Low melting point
Inertness
Astatine is primarily used in which field?
Agriculture
Medicine
Construction
Textile
How was Astatine first discovered?
Through electrolysis of seawater
By neutron bombardment of bismuth
Through mining
By fractional distillation of air
In the periodic table, Astatine is located in which period?
Period 4
Period 5
Period 6
Period 8
Before you leave, take our quick quiz to enhance your learning!

