What is the atomic number of Barium?
56
57
55
54
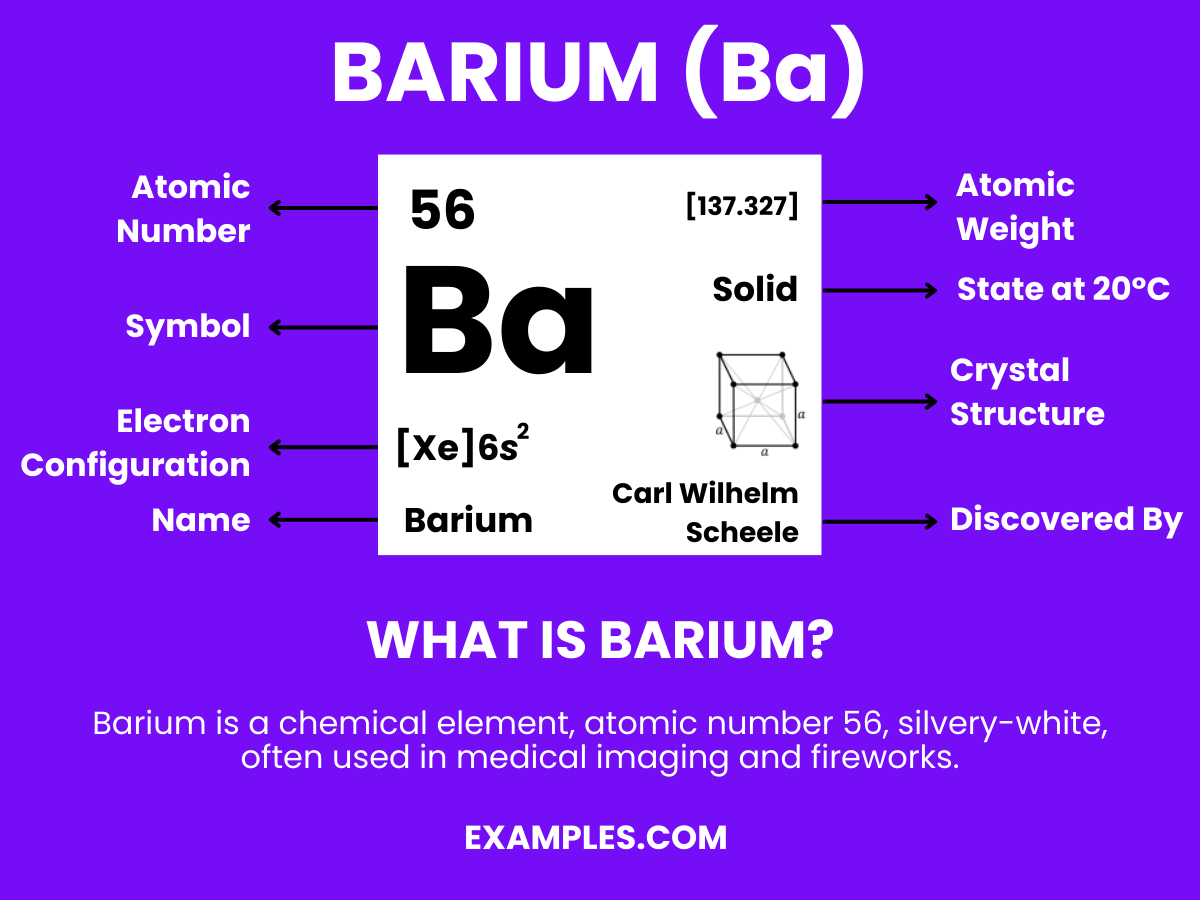
Barium plays a vital role in various scientific applications, intriguing both teachers and students alike. This guide aims to demystify Barium, highlighting its unique properties and practical uses in a classroom setting. By understanding Barium’s characteristics and applications, educators can enrich their teaching methodologies and spark students’ interest in chemistry. This comprehensive overview will provide teachers with the necessary tools to effectively incorporate Barium into their curriculum, ensuring a dynamic and engaging learning experience.
Barium is a chemical element with the symbol Ba and atomic number 56. It is a soft, silvery alkaline earth metal that, because of its high chemical reactivity, is never found in nature as a free element. Barium has several important applications, including in fireworks to create green colors, in oil and gas drilling fluids, and as a contrast agent in medical radiology. Barium compounds, such as barium sulfate and barium carbonate, are also used in the manufacturing of ceramics and glasses. Understanding Barium’s properties and uses can be a valuable addition to science education, particularly in demonstrating real-world chemical applications to students.
| Beryllium(Be) |
| Magnesium(Mg) |
| Calcium(Ca) |
| Strontium(Sr) |
| Radium(Ra) |
| Property | Description |
|---|---|
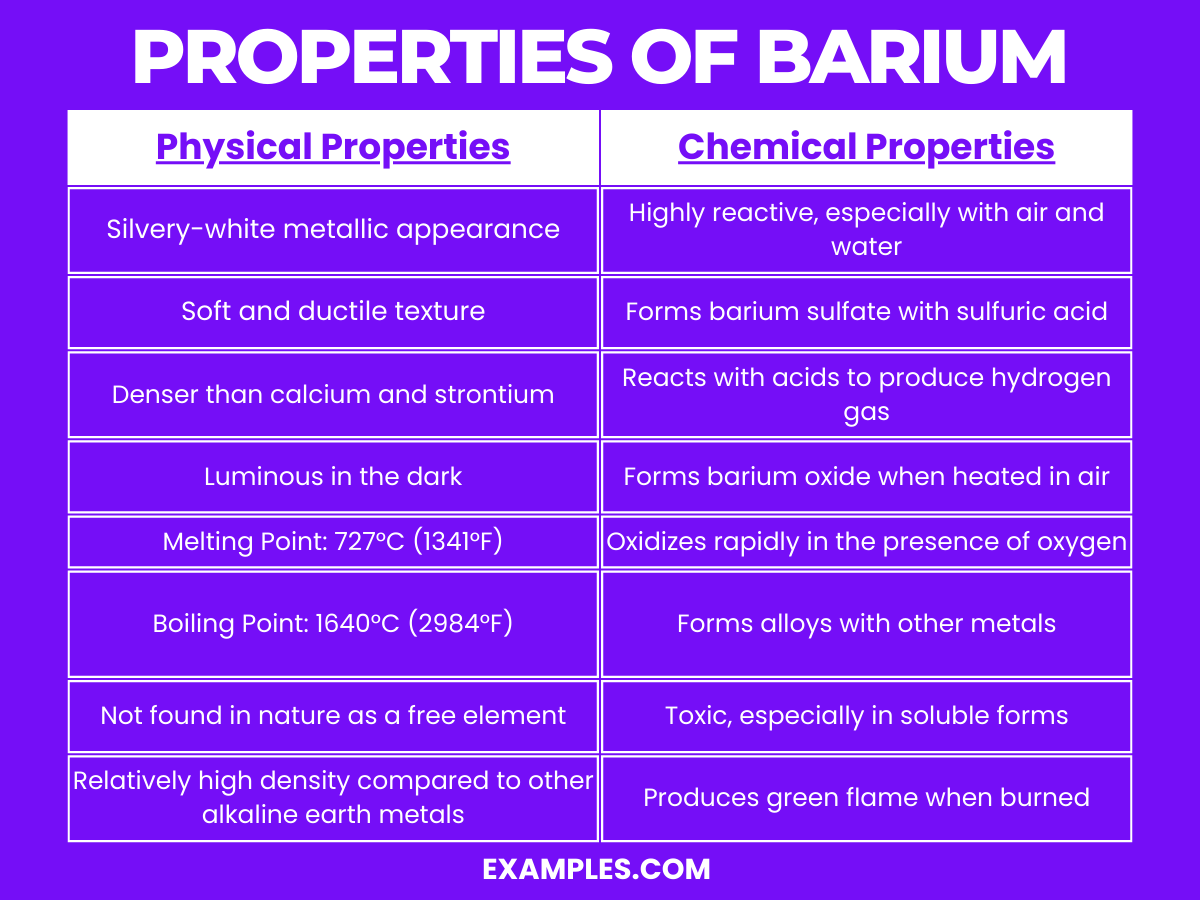
| Appearance | Silvery-white, lustrous metal |
| State at Room Temperature | Solid |
| Melting Point | 727°C |
| Boiling Point | 1897°C |
| Density | 3.51 g/cm³ |
| Malleability | Relatively soft, can be cut with a knife |
| Conductivity | Good electrical and thermal conductor |
| Solubility in Water | Insoluble, reacts with water to form barium hydroxide |
Barium is a chemical element with the symbol Ba and atomic number 56. It is part of the alkaline earth metal group, similar to calcium and magnesium, and exhibits several notable chemical properties:
| Property | Description / Value |
|---|---|
| Melting Point | 727°C (1341°F) |
| Boiling Point | 1845°C (3353°F) |
| Thermal Conductivity | 18.4 W/(m·K) |
| Specific Heat | 0.204 J/(g·K) at 25°C |
| Heat of Vaporization | 140.3 kJ/mol at boiling point |
| Heat of Fusion | 7.12 kJ/mol at melting point |
| Property | Description / Value |
|---|---|
| Phase at STP | Solid |
| Density | 3.62 g/cm³ at 20°C |
| Young’s Modulus | 13 GPa |
| Tensile Strength | 13 MPa |
| Mohs Hardness | 1.25 |
| Elastic Modulus | 9.6 GPa |
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Diamagnetic |
| Electrical Conductivity | 3.4 MS/m |
| Property | Description / Value |
|---|---|
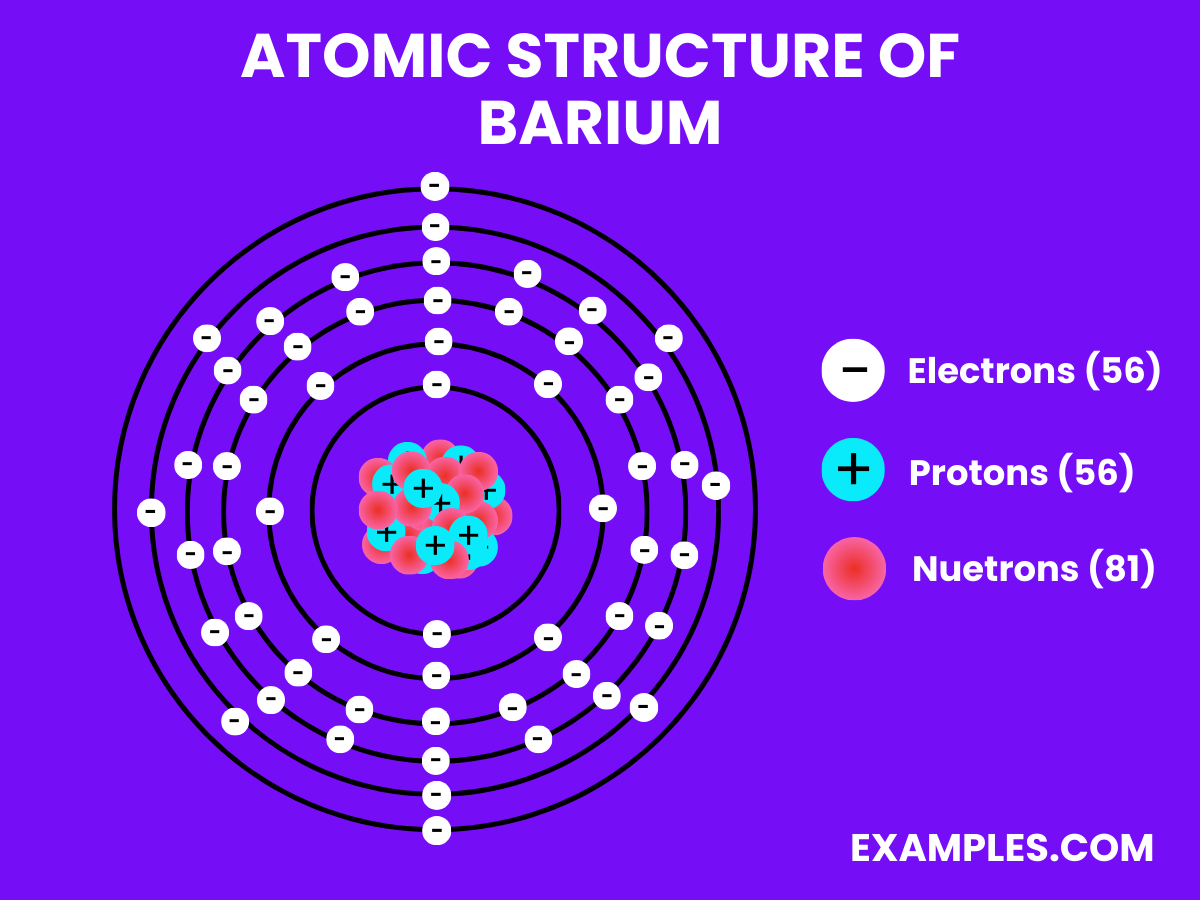
| Atomic Number | 56 |
| Atomic Mass | 137.327 u |
| Neutron Cross Section | 1.1 barns (for ^138Ba) |
| Isotopes | Natural barium is a mix of seven isotopes: ^130Ba, ^132Ba, ^134Ba, ^135Ba, ^136Ba, ^137Ba, and ^138Ba |
| Radioactivity | ^131Ba, ^133Ba, ^137mBa are among the notable radioactive isotopes, with ^137mBa being commonly used in medical and industrial applications |
Barium, a chemical element with diverse applications, forms several important compounds. Here are the top six barium compounds:
| Isotope | Symbol | Atomic Mass (u) | Half-Life |
|---|---|---|---|
| Barium-130 | ¹³⁰Ba | 129.9063 | Stable |
| Barium-132 | ¹³²Ba | 131.9051 | Stable |
| Barium-134 | ¹³⁴Ba | 133.9045 | Stable |
| Barium-135 | ¹³⁵Ba | 134.9057 | Stable |
| Barium-136 | ¹³⁶Ba | 135.9046 | Stable |
| Barium-137 | ¹³⁷Ba | 136.9058 | Stable |
| Barium-138 | ¹³⁸Ba | 137.9052 | Stable |
The most common isotope, Barium-138, accounts for approximately 71.7% of natural barium.
These isotopes are primarily used in scientific research and have various industrial applications.
The commercial production of barium involves several stages, primarily focusing on extracting barium from barite, a naturally occurring barium sulfate mineral.
The commercial production of barium must adhere to environmental and safety regulations due to the toxicity of some barium compounds. Handling and disposal of barium waste require careful management to prevent environmental contamination.
The health effects of barium depend on its forms and exposure levels. Barium compounds, when not handled properly, can pose health risks.
Barium exposure is usually occupational and related to the mining, processing, or handling of barium compounds. Safety measures and regulatory guidelines are essential to minimize health risks.
Barium compounds can impact the environment, especially when released from industrial processes or improper waste disposal.
Barium can cause gastrointestinal issues, respiratory problems, and affect heart rhythm when ingested in soluble forms. Barium sulfate used in medical imaging is generally safe.
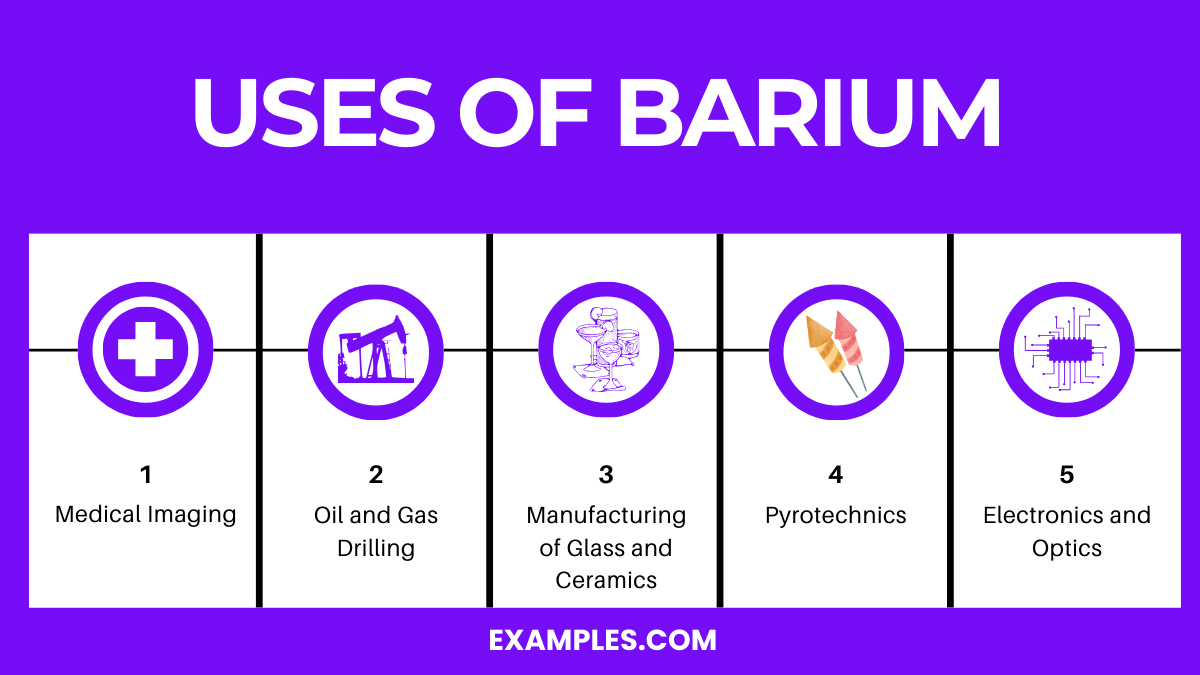
Barium is used in medical imaging, manufacturing glass and ceramics, oil and gas drilling, pyrotechnics for fireworks, and in electronics and optics.
Soluble barium compounds can be toxic, causing cardiovascular, respiratory, and neurological issues. However, barium sulfate used in medical procedures is not absorbed and is considered safe.
Foods generally have low barium levels. However, Brazil nuts, seaweed, fish, and certain leafy vegetables can contain higher amounts of naturally occurring barium.
Barium’s wide-ranging applications, from medical imaging to manufacturing, demonstrate its utility. While certain forms can be toxic, its regulated use in industries and healthcare is beneficial. Acknowledging both the uses and risks of barium is crucial for its effective and safe application.

Barium plays a vital role in various scientific applications, intriguing both teachers and students alike. This guide aims to demystify Barium, highlighting its unique properties and practical uses in a classroom setting. By understanding Barium’s characteristics and applications, educators can enrich their teaching methodologies and spark students’ interest in chemistry. This comprehensive overview will provide teachers with the necessary tools to effectively incorporate Barium into their curriculum, ensuring a dynamic and engaging learning experience.
Barium is a chemical element with the symbol Ba and atomic number 56. It is a soft, silvery alkaline earth metal that, because of its high chemical reactivity, is never found in nature as a free element. Barium has several important applications, including in fireworks to create green colors, in oil and gas drilling fluids, and as a contrast agent in medical radiology. Barium compounds, such as barium sulfate and barium carbonate, are also used in the manufacturing of ceramics and glasses. Understanding Barium’s properties and uses can be a valuable addition to science education, particularly in demonstrating real-world chemical applications to students.
Formula: Ba
Composition: A single barium atom.
Bond Type: Barium typically forms ionic bonds, due to its two outermost electrons.
Molecular Structure: Heavy, soft, silvery-white metal.
Electron Configuration: Fifty-six electrons, following the configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 4d¹⁰ 5s² 5p⁶ 6s².
Significance: Widely used in drilling fluids, paints, glassmaking, and as a contrast agent in medical radiography.
Role in Chemistry: Acts as a heavy metal in chemical reactions and is important in the petroleum industry.


Property | Description |
|---|---|
Appearance | Silvery-white, lustrous metal |
State at Room Temperature | Solid |
Melting Point | 727°C |
Boiling Point | 1897°C |
Density | 3.51 g/cm³ |
Malleability | Relatively soft, can be cut with a knife |
Conductivity | Good electrical and thermal conductor |
Solubility in Water | Insoluble, reacts with water to form barium hydroxide |
Barium is a chemical element with the symbol Ba and atomic number 56. It is part of the alkaline earth metal group, similar to calcium and magnesium, and exhibits several notable chemical properties:
Reactivity with Water: Barium reacts with water to produce barium hydroxide (Ba(OH)₂) and hydrogen gas (H₂). This reaction is fairly vigorous and releases heat, highlighting its reactive nature.
Formation of Compounds: Barium readily forms compounds with other elements, particularly halogens, to form barium salts like barium chloride (BaCl₂) and barium sulfate (BaSO₄).
Role in Oxidation States: In compounds, barium most commonly exhibits a +2 oxidation state, losing its two outermost electrons to form Ba²⁺ ions.
Radioactive Isotopes: Some isotopes of barium are radioactive. Barium-130 and Barium-132 can undergo K-capture, leading to the formation of xenon.
Use in Removing Gases: Barium can combine with gases such as oxygen and sulfur at high temperatures, making it useful in vacuum tubes to remove unwanted gases.
Color in Flame Test: Barium compounds impart a green color in a flame test, which is a characteristic feature used in identifying barium.
Low Reactivity with Air: Unlike some other alkaline earth metals, barium does not react as quickly with air at room temperature but can oxidize when heated.
Solubility in Acids: Barium is soluble in most acids, including hydrochloric acid and sulfuric acid, but the sulfate forms are insoluble in water.
Property | Description / Value |
|---|---|
Melting Point | 727°C (1341°F) |
Boiling Point | 1845°C (3353°F) |
Thermal Conductivity | 18.4 W/(m·K) |
Specific Heat | 0.204 J/(g·K) at 25°C |
Heat of Vaporization | 140.3 kJ/mol at boiling point |
Heat of Fusion | 7.12 kJ/mol at melting point |
Property | Description / Value |
|---|---|
Phase at STP | Solid |
Density | 3.62 g/cm³ at 20°C |
Young’s Modulus | 13 GPa |
Tensile Strength | 13 MPa |
Mohs Hardness | 1.25 |
Elastic Modulus | 9.6 GPa |
Property | Description / Value |
|---|---|
Magnetic Susceptibility | Diamagnetic |
Electrical Conductivity | 3.4 MS/m |
Property | Description / Value |
|---|---|
Atomic Number | 56 |
Atomic Mass | 137.327 u |
Neutron Cross Section | 1.1 barns (for ^138Ba) |
Isotopes | Natural barium is a mix of seven isotopes: ^130Ba, ^132Ba, ^134Ba, ^135Ba, ^136Ba, ^137Ba, and ^138Ba |
Radioactivity | ^131Ba, ^133Ba, ^137mBa are among the notable radioactive isotopes, with ^137mBa being commonly used in medical and industrial applications |
Barium, a chemical element with diverse applications, forms several important compounds. Here are the top six barium compounds:
Formula: BaSO₄
Preparation: BaCl₂ + Na₂SO₄ → BaSO₄ + 2NaCl
Uses: Used in medical imaging for X-ray examinations and as a pigment in paints.
Formula: Ba(NO₃)₂
Preparation: Ba(OH)₂ + 2HNO₃ → Ba(NO₃)₂ + 2H₂O
Uses: Commonly used in fireworks to create green colors and in the pyrotechnics industry.
Formula: BaCl₂
Preparation: Ba + Cl₂ → BaCl₂
Uses: Used in heat treatment baths for steel and as a test for sulfate ions.
Formula: BaCO₃
Preparation: Ba(OH)₂ + CO₂ → BaCO₃ + H₂O
Uses: Used in the production of glass and ceramics, and as a rodenticide.
Formula: BaO
Preparation: 2Ba + O₂ → 2BaO
Uses: Utilized in the manufacture of lubricants, ceramics, and certain types of glass.
Formula: Ba(OH)₂
Preparation: BaO + H₂O → Ba(OH)₂
Uses: Employed in the purification of sugar and as a stabilizer in plastics.
Isotope | Symbol | Atomic Mass (u) | Half-Life |
|---|---|---|---|
Barium-130 | ¹³⁰Ba | 129.9063 | Stable |
Barium-132 | ¹³²Ba | 131.9051 | Stable |
Barium-134 | ¹³⁴Ba | 133.9045 | Stable |
Barium-135 | ¹³⁵Ba | 134.9057 | Stable |
Barium-136 | ¹³⁶Ba | 135.9046 | Stable |
Barium-137 | ¹³⁷Ba | 136.9058 | Stable |
Barium-138 | ¹³⁸Ba | 137.9052 | Stable |
The most common isotope, Barium-138, accounts for approximately 71.7% of natural barium.
These isotopes are primarily used in scientific research and have various industrial applications.

Barium Sulfate (BaSO₄): Used as a contrast agent in X-ray imaging and CT scans. It’s ingested to coat the lining of the stomach, intestines, or esophagus, enhancing the clarity of the images.
Barium Sulfate: Added to drilling muds in the oil and gas industry. It increases the mud’s density, stabilizing the borehole and lubricating the drill bit.
Barium Carbonate (BaCO₃): Used to remove impurities and enhance the optical qualities in glass manufacturing. It’s also utilized in producing certain ceramic glazes.
Barium Nitrate (Ba(NO₃)₂): Gives fireworks a green color. Barium compounds are used in pyrotechnics to create colors and effects due to their ability to emit light when burned.
Barium Titanate (BaTiO₃): Employed in electronics for its dielectric properties. Used in capacitors, electromechanical transducers, and in optics for its refractive index.
The commercial production of barium involves several stages, primarily focusing on extracting barium from barite, a naturally occurring barium sulfate mineral.
Barite is mined through surface or underground methods. The ore is drilled, blasted, and then transported for further processing.
The extracted barite ore is crushed and ground into a fine powder to increase the surface area for the subsequent processing steps.
The ground barite is subjected to gravity separation or flotation processes to separate barium sulfate from impurities based on their physical properties.
Purified barium sulfate is then converted into different barium compounds. For instance, barium carbonate is produced by treating barium sulfate with carbon at high temperatures.
Barium chloride is made by reacting barium sulfate with calcium chloride, and other compounds are synthesized using similar chemical reactions.
The produced barium compounds are rigorously tested for purity and quality. After meeting the required standards, they are packaged and distributed for various industrial uses.
The commercial production of barium must adhere to environmental and safety regulations due to the toxicity of some barium compounds. Handling and disposal of barium waste require careful management to prevent environmental contamination.
The health effects of barium depend on its forms and exposure levels. Barium compounds, when not handled properly, can pose health risks.
Barium Sulfate in Medical Imaging: When used as a contrast agent in X-ray imaging, barium sulfate is safe as it doesn’t get absorbed into the body.
Gastrointestinal Issues: Ingestion of certain barium compounds can cause vomiting, diarrhea, and stomach cramps.
Respiratory Problems: Inhalation of barium dust or fumes can lead to breathing difficulties and irritation of the lungs.
Cardiovascular Effects: High levels of soluble barium compounds can affect the heart, potentially leading to changes in blood pressure and heart rhythm disturbances.
Muscle Weakness and Cramps: Excessive exposure to barium can cause muscle weakness and cramping, as barium interferes with potassium channels in the body.
Neurological Impact: In extreme cases, high barium exposure may lead to changes in nerve reflexes, headaches, or even paralysis.
Barium exposure is usually occupational and related to the mining, processing, or handling of barium compounds. Safety measures and regulatory guidelines are essential to minimize health risks.
Barium compounds can impact the environment, especially when released from industrial processes or improper waste disposal.
Barium can accumulate in soils and water bodies, especially near mining sites or industrial areas. This can affect the local ecosystem and potentially enter the human food chain.
High concentrations of barium in water can be toxic to fish and aquatic organisms. It can disrupt their osmotic balance and metabolic functions.
Barium in the soil can affect plant growth. Certain barium compounds, when present in high amounts, can be toxic to plants, hindering their development and growth.
Airborne barium particles, resulting from industrial activities, can degrade air quality. While not a major greenhouse gas, it contributes to particulate pollution.
Barium compounds can persist in the environment for long periods, especially in sediment and soils, posing long-term environmental risks.
Barium can cause gastrointestinal issues, respiratory problems, and affect heart rhythm when ingested in soluble forms. Barium sulfate used in medical imaging is generally safe.
Barium is used in medical imaging, manufacturing glass and ceramics, oil and gas drilling, pyrotechnics for fireworks, and in electronics and optics.
Soluble barium compounds can be toxic, causing cardiovascular, respiratory, and neurological issues. However, barium sulfate used in medical procedures is not absorbed and is considered safe.
Foods generally have low barium levels. However, Brazil nuts, seaweed, fish, and certain leafy vegetables can contain higher amounts of naturally occurring barium.
Barium’s wide-ranging applications, from medical imaging to manufacturing, demonstrate its utility. While certain forms can be toxic, its regulated use in industries and healthcare is beneficial. Acknowledging both the uses and risks of barium is crucial for its effective and safe application.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Barium?
56
57
55
54
What is the main hazard associated with barium if ingested in soluble forms?
It is radioactive.
It is toxic.
It is an irritant.
Barium is commonly used in the production of:
Plastics
Ceramics
Fireworks
Pharmaceuticals
Before you leave, take our quick quiz to enhance your learning!

