Who is credited with the discovery of the structure of Benzene?
Marie Curie
August Kekule
Dmitri Mendeleev
Linus Pauling

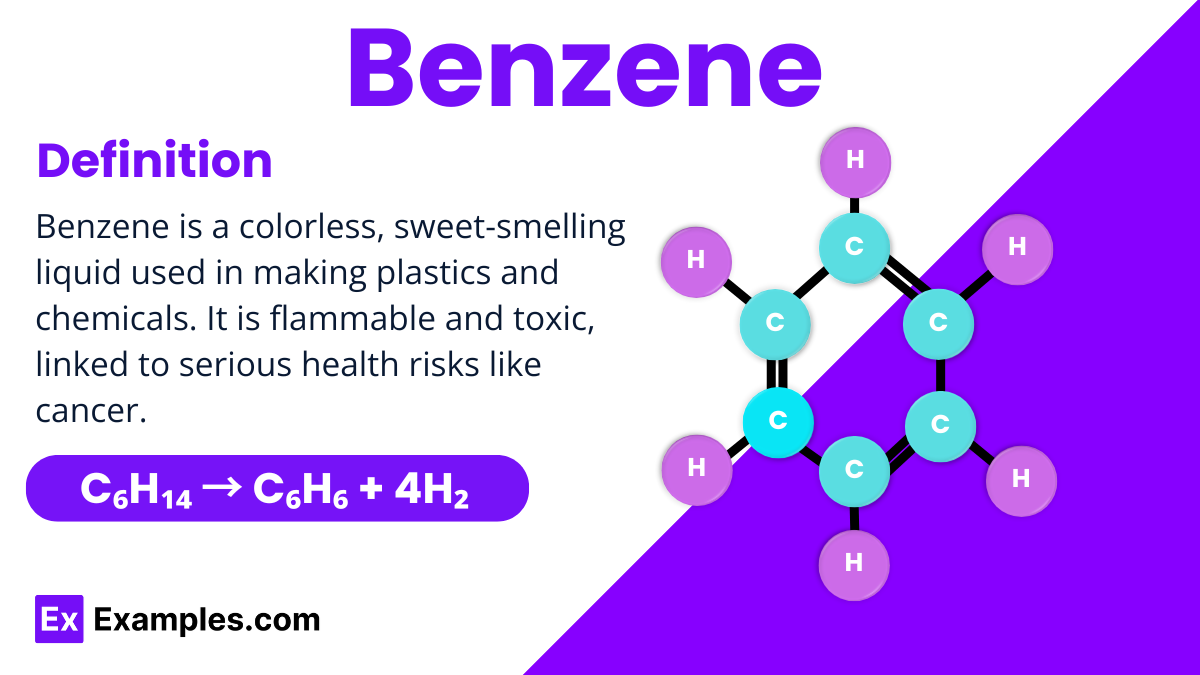
Benzene is a fundamental organic compound in chemistry, known for its simple yet unique structure consisting of six carbon atoms forming a ring, each bonded to a hydrogen atom. This arrangement is often depicted as a hexagon with alternating double bonds, making it a perfect example of aromatic stability. Benzene is colorless, highly flammable, and has a distinct sweet smell, used extensively in manufacturing a wide range of products, from plastics to synthetic fibers.
| Property | Value |
|---|---|
| Formula | C₆H₆ |
| Name | Benzene |
| Alternate Names | Benzin, Benzine, Benzol, Benzole, Benzolene, Cyclohexatriene, Phenyl Hydride, Pyrobenzole |
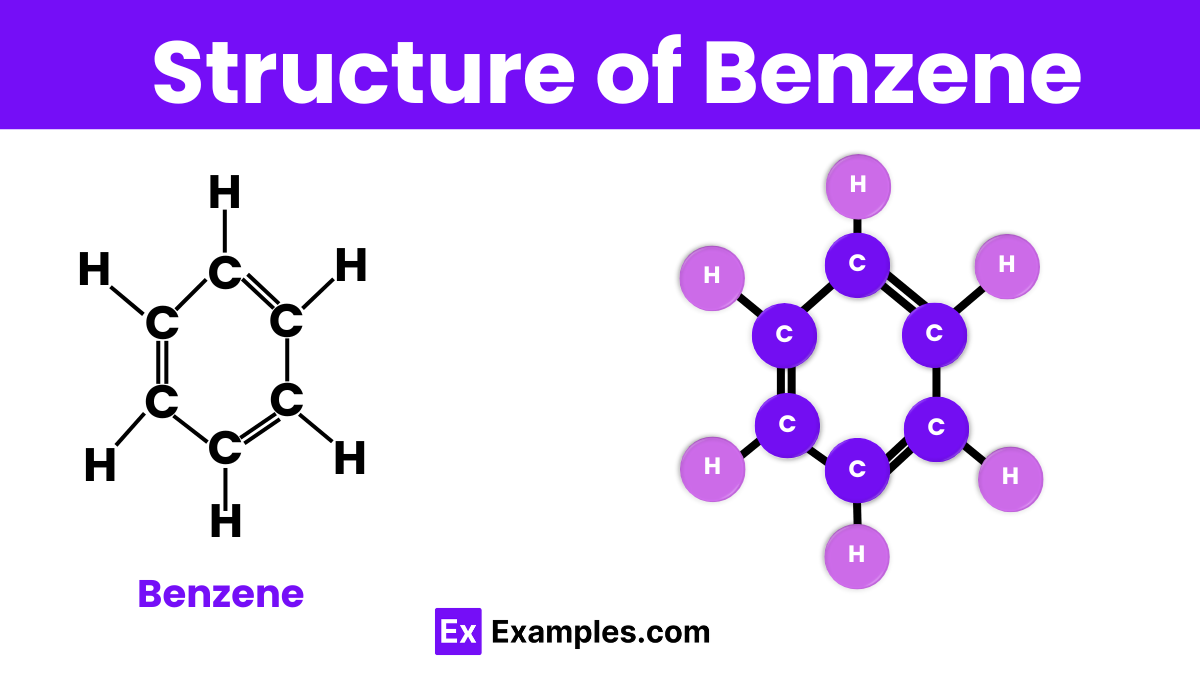
Benzene is a fascinating molecule with a unique structure that makes it stand out in organic chemistry. It consists of six carbon atoms arranged in a hexagonal ring, connected by alternating single and double bonds. This arrangement is known as aromatic due to its stability and special bonding. Each carbon atom in the ring is also bonded to one hydrogen atom. The beauty of benzene’s structure lies in its symmetry and the equal length of all its carbon-carbon bonds, which are shorter than typical single bonds but longer than double bonds. This configuration allows benzene to be exceptionally stable and widely used in various chemical processes.
Benzene can be prepared through several methods, but one of the most common is the catalytic reforming of alkanes. This process involves converting straight-chain hydrocarbons from petroleum into benzene, among other products. A typical reaction might involve heating an alkane like hexane in the presence of a platinum catalyst, which facilitates the rearrangement and eventual formation of benzene.A basic chemical equation illustrating this transformation is:
This equation shows hexane (C₆H₁₄) being converted into benzene (C₆H₆) and hydrogen gas (H₂). The production of benzene in this manner highlights its importance in industrial applications, where it serves as a building block for many chemicals and synthetic materials.
| Property | Description |
|---|---|
| Appearance | Clear, colorless liquid |
| Smell | Sweet, aromatic |
| Boiling Point | 80.1°C (176°F) |
| Melting Point | 5.5°C (41.9°F) |
| Density | 0.8765 g/cm³ (at 20°C) |
| Solubility in Water | Insoluble |
| Vapor Pressure | 12.7 kPa (at 25°C) |
| Molecular Weight | 78.11 g/mol |
| Flammability | Highly flammable |
| Property | Value |
|---|---|
| CAS Registry Number | 71-43-2 |
| Beilstein Number | 969212 |
| PubChem Compound ID | 241 |
| SMILES Identifier | C1=CC=CC=C1 |
| InChI Identifier | InChI=1/C6H6/c1-2-4-6-5-3-1/h1-6H |
| RTECS Number | CY1400000 |
| MDL Number | MFCD00003009 |
| Property | Value |
|---|---|
| NFPA Health Rating | 2 |
| NFPA Fire Rating | 3 |
| NFPA Reactivity Rating | 0 |
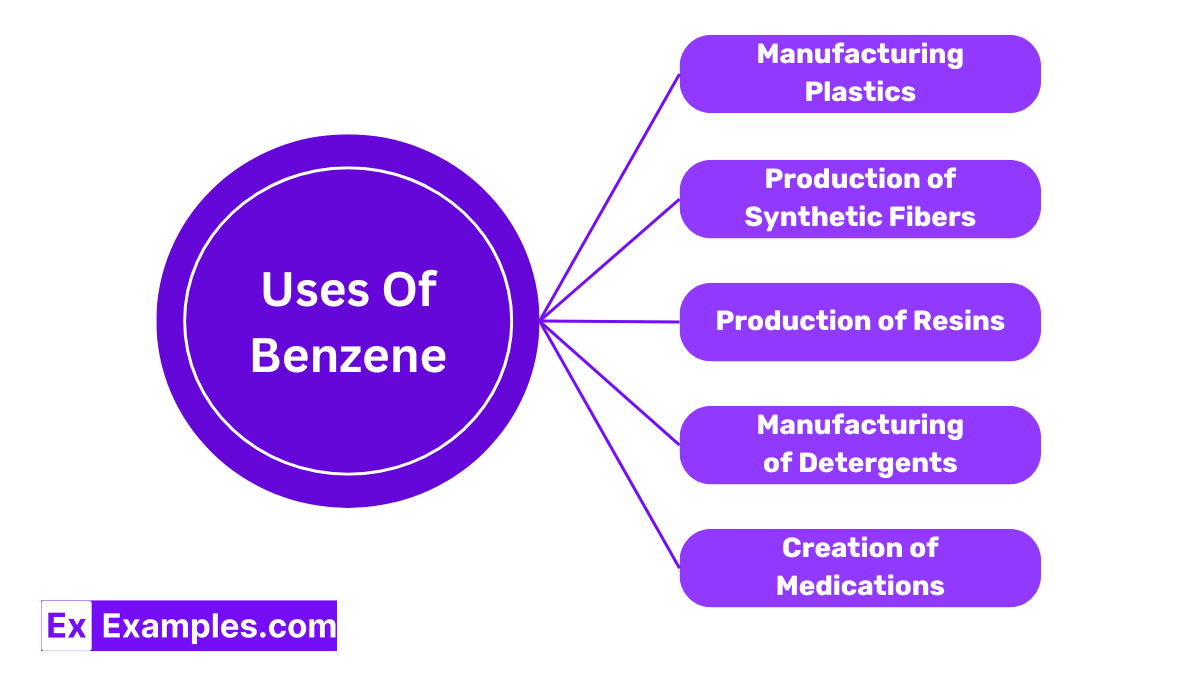
Benzene is a crucial ingredient in the production of polystyrene, a type of plastic used widely in everything from packaging to insulation materials. Its ability to polymerize easily makes it invaluable in creating durable and versatile plastic products.
Benzene is used to manufacturing synthetic fibers like nylon and polyester. These fibers are essential for producing a wide range of products, from clothing to home furnishings, due to their strength and resistance to shrinking and stretching.
Benzene is also used in the synthesis of various resins, which are substances used as the basis for coatings and adhesives. These resins enhance the durability and effectiveness of paints and varnishes, ensuring they adhere better and last longer.
Benzene derivatives are employed to enhance the cleaning power of laundry and dish washing products. These derivatives help break down stains and grease, making them effective cleaning agents.
Benzene is a building block for some pharmaceuticals, contributing to the synthesis of drugs used to treat various medical conditions. Its chemical properties allow for the creation of complex drug formulations, improving health care and treatment options.
Benzene is highly used in the manufacturing of a wide range of products including plastics, synthetic rubber, and dyes. This makes it a cornerstone chemical in the industrial sector, driving innovation and production across various fields.
Due to its critical role in many manufacturing processes, benzene helps stimulate economic growth within the chemical industry and downstream sectors such as automotive, construction, and electronics. Its widespread use contributes significantly to economic development and job creation.
Benzene derivatives play a vital role in pharmaceutical development, leading to the creation of new medications and treatments. These advancements improve healthcare outcomes and contribute to the well-being of populations worldwide.
Benzene is used in the production of everyday items like lubricants, improving their effectiveness and efficiency. This enhances consumer products, making daily chores easier and supporting modern lifestyles.
Benzene is famous for its use in making plastics, resins, and synthetic fibers, and for being a significant health hazard.
Benzene exposure is primarily linked to an increased risk of leukemia and other blood-related cancers.
Benzene is banned in many places due to its severe toxicity and strong links to cancer, making it a significant public health risk.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Who is credited with the discovery of the structure of Benzene?
Marie Curie
August Kekule
Dmitri Mendeleev
Linus Pauling
What is the bond angle between the carbon atoms in Benzene?
90°
109.5°
120°
180°
What is the characteristic feature of Benzene\'s structure?
Single bonds only
Double bonds only
Alternating single and double bonds
Delocalized pi electrons
Which type of reaction is Benzene most likely to undergo?
Addition
Elimination
Substitution
Polymerization
What is the result of the nitration of Benzene?
Benzene sulfonic acid
Nitrobenzene
Chlorobenzene
Phenol
What is the typical catalyst used in the hydrogenation of Benzene?
Iron
Nickel
Copper
Platinum
What type of molecular orbital system does Benzene have?
Conjugated system
Isolated pi bonds
Sigma bonds only
Antibonding orbitals
What is the resonance energy of Benzene?
100 kJ/mol
150 kJ/mol
200 kJ/mol
250 kJ/mol
Which is not a derivative of Benzene?
Toluene
Aniline
Phenol
Ethylene
What is the boiling point of Benzene?
80°C
100°C
120°C
60°C
Before you leave, take our quick quiz to enhance your learning!

