Bohrium was named in honor of which famous scientist?
Albert Einstein
Niels Bohr
Dmitri Mendeleev
Marie Curie
Bohrium, a synthetic element with a heavyweight presence in the periodic table, stands as a testament to human ingenuity in the realm of chemistry. This guide embarks on a fascinating journey to uncover the definition, meaning, and myriad uses of Bohrium, along with an exploration of its compounds. As we delve into the atomic intricacies of Bohrium, we unlock the door to understanding its role in scientific advancements and its potential applications. With Bohrium’s elusive nature and its contribution to the field of chemistry, this introduction serves as a gateway to discovering the untapped potentials of one of the most intriguing elements on the periodic table.
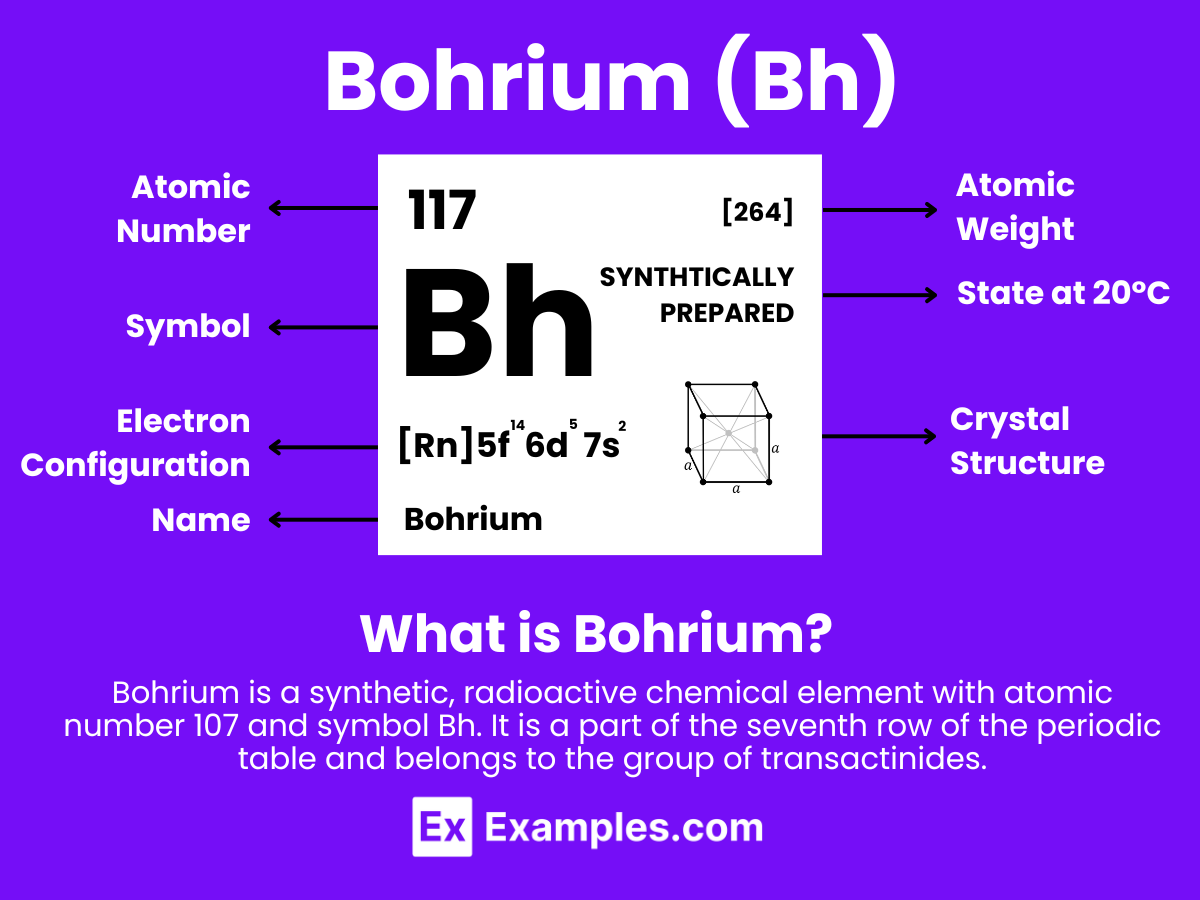
Bohrium is a chemical element with the symbol Bh and atomic number 107. It is a synthetic element, and thus not found in nature. Bohrium is part of the seventh row of the periodic table and belongs to the d-block. It is a member of the transactinide elements and the group 7 elements, sharing this group with manganese (Mn), technetium (Tc), and rhenium (Re).
Because Bohrium is a synthetic element, it is produced in particle accelerators through the collision of lighter atomic nuclei. For example, it can be created by the fusion of bismuth and chromium atoms. Bohrium was first synthesized in 1981 by a team of scientists at the Gesellschaft für Schwerionenforschung (GSI) in Darmstadt, Germany.
Bohrium, in contrast to hydrogen, is a metallic element with theoretical characteristics suggesting it could display unique properties, including potential instability in solid or liquid form due to its radioactive nature. The behavior of bohrium at the atomic and molecular levels significantly diverges from that of hydrogen, given its position as a superheavy element in the periodic table and its anticipated metallic characteristics.
Atomic Level: Each bohrium atom (Bh) contains 107 protons in its nucleus and is expected to have 107 electrons orbiting around it. The electron configuration of bohrium is predicted to be [Rn] 5f¹⁴ 6d⁵ 7s², suggesting it has a complex electron configuration with potential for various oxidation states, similar to other elements in group 7 of the periodic table. This indicates a certain level of chemical reactivity and the possibility for forming compounds under specific conditions, albeit theoretical due to bohrium’s extreme radioactivity.
Molecular Formation: Unlike hydrogen, which forms simple molecules like H₂ through covalent bonding, bohrium would not form molecules in a similar manner due to its metallic nature. If it were possible to observe bohrium in a bulk form, theoretical predictions suggest that bohrium atoms might be part of an unknown metallic lattice structure. This hypothetical structure would involve metallic bonding, where electrons are delocalized over many bohrium atoms, differing fundamentally from the discrete electron sharing seen in hydrogen’s covalent bonds. Given bohrium’s highly radioactive nature and very short half-life, any metallic form it might take would be ephemeral and challenging to study directly.
Here’s a table detailing the physical properties of Bohrium. Due to the element’s synthetic and highly unstable nature, these properties are largely theoretical and based on scientific predictions rather than direct observation.
| Property | Description |
|---|---|
| Atomic Number | 107 |
| Atomic Mass | The most stable isotope, Bh-270, has an atomic mass of approximately 270 u. |
| State at Room Temperature | Presumed to be a solid, though never observed in macroscopic quantities due to rapid decay. |
| Density | Unknown; predicted to be high given its position among the heavier elements in the periodic table. |
| Melting Point | Theoretical; expected to be high, comparable to other transactinide elements but not directly measured. |
| Boiling Point | Unknown; highly radioactive and short-lived isotopes make direct measurement impractical. |
| Appearance | Theoretical; no color or appearance has been directly observed due to its instability and rarity. |
| Crystal Structure | Predicted to be metallic with an unknown crystalline structure; purely speculative. |
Bohrium is a synthetic element with the symbol Bh and atomic number 107. As a member of the Group 7 elements in the periodic table, it shares properties with its lighter congeners, manganese (Mn), technetium (Tc), and rhenium (Re). However, due to its highly unstable and radioactive nature, with a half-life that makes extensive laboratory study difficult, much of what is known about Bohrium’s chemical properties is theoretical, derived from computer simulations and comparison to other Group 7 elements.
Bohrium’s electronic configuration is theoretically predicted to be [Rn] 5f¹⁴ 6d⁵7s², similar to other group 7 elements. This configuration suggests that Bohrium would exhibit typical transition metal chemistry, which is characterized by the formation of complex compounds and the ability to exist in multiple oxidation states.
Bohrium is expected to exhibit a variety of oxidation states, with +7 being the most stable and characteristic, similar to manganese (Mn), technetium (Tc), and rhenium (Re). However, other oxidation states, including +3, +4, and +5, might also be possible and could be stable in certain compounds. The +7 oxidation state, in particular, could lead to the formation of oxides and halides analogous to those formed by its lighter homologues, such as ReO4^− (perrhenate ion) and TcO4^− (pertechnetate ion).
| Property | Value | Notes |
|---|---|---|
| Atomic Number | 107 | |
| Atomic Mass | [270] | Most stable isotope; mass numbers of known isotopes range from 260 to 274. |
| Melting Point | Unknown | Predicted to be similar to that of rhenium (~3186°C). |
| Boiling Point | Unknown | Predicted to be similar to that of rhenium (~5630°C). |
| Density | Unknown | Predicted to be around 37 g/cm³, based on extrapolations. |
| Standard State | Presumably Solid | Based on its position in the periodic table. |
| Heat of Fusion | Unknown | |
| Heat of Vaporization | Unknown | |
| Specific Heat Capacity | Unknown | |
| Thermal Conductivity | Unknown | |
| Thermal Expansion | Unknown |
| Property | Value | Notes |
|---|---|---|
| Crystal Structure | Unknown | Predicted to be hexagonal close-packed (hcp), similar to rhenium. |
| Electrical Conductivity | Unknown | Expected to be a poor conductor, similar to other transactinide elements. |
| Magnetic Properties | Unknown | Predicted to be paramagnetic, similar to rhenium. |
| Hardness | Unknown | |
| Elastic Modulus | Unknown | |
| Poisson Ratio | Unknown | |
| Ductility | Unknown | |
| Malleability | Unknown | |
| Corrosion Resistance | Unknown | Predicted to be relatively high, based on group trends. |
The table below outlines the theoretical electromagnetic properties of Bohrium, based on scientific predictions. Direct observations are not available due to the element’s synthetic nature and the rapid decay of its isotopes.
| Property | Description |
|---|---|
| Electron Configuration | Predicted to be [Rn] 5f¹⁴ 6d⁵ 7s², indicating a complex behavior typical of heavy elements. |
| Oxidation States | +3, +5, +7 are theorized, with +7 being the most stable but highly theoretical. |
| Electrical Conductivity | Assumed to be metallic, indicating good conductivity, though specific values are not determined. |
| Magnetic Properties | Not directly known; theoretical predictions suggest it could be paramagnetic or diamagnetic. |
This table presents the known nuclear properties of Bohrium, which are derived from experimental observations of its isotopes and theoretical models.
| Property | Description |
|---|---|
| Isotopes | Known isotopes range from Bh-260 to Bh-274, with Bh-270 being among the most stable. |
| Half-Life | The half-lives of Bohrium isotopes vary; Bh-270, for example, has a half-life of about 61 seconds. |
| Decay Modes | Primarily undergoes alpha decay; some isotopes may undergo spontaneous fission. |
| Production Method | Produced in particle accelerators by bombarding bismuth or lead with chromium or nickel nuclei. |
| Stability | All isotopes are highly unstable due to their large atomic numbers and the resulting radioactive decay. |
| Nuclear Shell Model | Predicted to conform to the shell model, which explains its nuclear stability relative to other elements. |
The preparation of Bohrium (Bh) involves nuclear reactions using particle accelerators. Since Bohrium does not occur naturally, it is produced by the collision of lighter atomic nuclei. The most common method for producing Bohrium involves bombarding a target of a heavier element with ions of a lighter element. The general process can be described by the following representative nuclear reactions:
These reactions are carried out in particle accelerators, where the speed and energy of the ion beams can be carefully controlled to facilitate the fusion of the atomic nuclei. The production of Bohrium is highly challenging due to the need for precise conditions and the element’s rapid decay. The isotopes of Bohrium produced in these reactions have very short half-lives, making immediate detection and analysis critical.
Bohrium Oxide (BhO₃)
Equation: 2Bh+3O₂ → 2BhO₃
Description: A theoretical oxide where Bohrium is expected to combine with oxygen in a +6 oxidation state. Its properties and stability are speculative, drawing parallels with rhenium oxide (ReO3).
Bohrium Chloride (BhCl₆)
Equation: Bh+ 3Cl₂ → BhCl₆
Description: Analogous to rhenium(VI) chloride (ReCl6), Bohrium chloride is predicted to form through direct reaction with chlorine, suggesting potential use in hypothetical chemical studies involving high oxidation states of Bohrium.
Bohrium Sulfide (BhS₂)
Equation: Bh+2S → BhS₂
Description: A speculated sulfide of Bohrium, analogous to rhenium sulfide (ReS2). Its formation implies Bohrium’s ability to bond with sulfur, potentially exhibiting similar properties to those of other Group 7 metal sulfides.
Bohrium Fluoride (BhF₆)
Equation: Bh+3 F₂ → BhF₆
Description: Theoretical fluoride of Bohrium, expected to form a volatile hexafluoride similar to rhenium(VI) fluoride (ReF6), emphasizing its possible reactivity with halogens in high oxidation states.
Bohrium Hydride (BhH₂)
Equation: Bh+H₂ →BhH₂
Description: A speculative compound, assuming Bohrium can react with hydrogen to form a dihydride. Its characteristics and existence remain purely theoretical, potentially following the trends of heavy metal hydrides within Group 7 elements.
Bohrium Iodide (BhI₆)
Equation: Bh+3I₂ → BhI₆
Description: Predicted to be analogous to rhenium(VI) iodide (ReI6), this compound suggests a reaction between Bohrium and iodine in a high oxidation state. The stability and properties of such a compound are subjects of speculation, given the chemical behavior of its lighter homologues in the periodic table
| Isotope | Half-Life | Decay Mode(s) | Notes |
|---|---|---|---|
| Bh-260 | Unknown | Alpha decay | |
| Bh-261 | ~11.8 ms | Alpha decay | |
| Bh-262 | ~102 ms | Alpha decay | |
| Bh-262m | ~8 ms | Isomeric transition (IT), Alpha decay | Meta-stable state |
| Bh-263 | ~0.1 s | Alpha decay | |
| Bh-264 | ~0.44 s | Alpha decay | |
| Bh-265 | ~0.9 s | Alpha decay | |
| Bh-266 | ~2.3 s | Alpha decay | |
| Bh-267 | ~17 s | Alpha decay | |
| Bh-270 | ~61 s | Alpha decay | Most stable isotope; existence of longer-lived isotopes has been suggested but not confirmed. |
| Bh-272 | Unknown | Alpha decay | Predicted, not yet confirmed. |
| Bh-274 | Unknown | Alpha decay | Predicted, not yet confirmed. |
Bohrium is a synthetic element that is not found in nature and is produced in particle accelerators through the fusion of smaller atomic nuclei. The production process involves highly sophisticated equipment and precise conditions to achieve the fusion, leading to the creation of a few atoms of Bohrium at a time. Here’s how Bohrium is typically produced:
Bohrium is a superheavy synthetic element that exists for only a short period due to its highly radioactive nature. As of now, Bohrium has no practical applications outside of scientific research due to its short half-life and the difficulty in producing it in significant quantities.
Bohrium, a synthetic element shrouded in mystery, epitomizes the pinnacle of scientific exploration into the periodic table’s far reaches. Its production and study, though fraught with challenges due to its radioactivity and ephemeral existence, offer invaluable insights into nuclear physics and chemistry’s frontiers. While Bohrium’s practical applications remain theoretical, its contribution to scientific knowledge is undeniable, expanding our understanding of superheavy elements and the fundamental nature of matter.
Text prompt
Add Tone
Production of Bohrium
Applications of Bohrium
Electrons (105)
Neutrons (163)
Protons (105)
Bohrium was named in honor of which famous scientist?
Albert Einstein
Niels Bohr
Dmitri Mendeleev
Marie Curie
What is the symbol for bohrium?
Bh
Bm
Bo
Bi
Bohrium is classified as what type of element?
Alkali metal
Alkaline earth metal
Transition metal
Noble gas
How was bohrium first synthesized?
Neutron bombardment
Fusion of lighter nuclei
Chemical reaction
Electrolysis
What is the most stable isotope of bohrium?
Bh-267
Bh-270
Bh-273
Bh-278
Bohrium is part of which period in the periodic table?
Period 6
Period 7
Period 8
Period 9
Bohrium has how many electrons in its outer shell?
5
6
7
8
Bohrium is primarily used for:
Medical applications
Industrial catalysts
Research purposes
Consumer products
Which laboratory successfully synthesized bohrium for the first time?
Lawrence Berkeley National Laboratory
Joint Institute for Nuclear Research
GSI Helmholtz Centre for Heavy Ion Research
Fermi National Accelerator Laboratory
Bohrium is expected to have similar chemical properties to which element?
Iron
Rhenium
Tungsten
Platinum
Before you leave, take our quick quiz to enhance your learning!

