Which civilization is known for starting the Bronze Age?
Egyptians
Mesopotamians
Greeks
Romans

Bronze is a durable metallic compound with a long history in various cultures, valued for its strength and beauty. It’s crafted through a chemical process that combines copper and tin, along with small amounts of other metals like aluminum, manganese, nickel, or zinc to create different types of bronze with unique properties. This blend makes bronze harder than pure copper, giving it a wide range of applications from making coins and medals to constructing statues and musical instruments. In chemistry, bronze is fascinating because it demonstrates how combining elements can create a material with new characteristics, including resistance to corrosion and a distinctive, golden-brown color.
Bronze is an alloy, which means it is made by melting and mixing two or more elements together. In the case of bronze, the primary elements are copper and tin. Copper, the main component, gives bronze its strength and ductility, allowing it to be easily shaped and molded. Tin, added in smaller amounts, enhances the hardness and melting point of the alloy, making it more resistant to wear and tear. The structure of bronze can vary depending on the exact ratio of copper to tin, leading to different types of bronze suited for various applications. Some bronzes may also include small amounts of other elements like zinc, lead, or phosphorus to alter its properties, such as improving its ability to cast or increasing its resistance to corrosion.
The preparation of bronze involves melting copper and tin together. First, copper is heated until it melts. The melting point of copper is about 1,984 degrees Fahrenheit (1,085 degrees Celsius). Once the copper is in its liquid form, tin is added to the molten copper. Tin melts at a lower temperature, around 449 degrees Fahrenheit (231 degrees Celsius), so it easily mixes with the molten copper. The typical ratio is about 88% copper to 12% tin, but this can vary depending on the desired properties of the bronze.
The chemical equation for the reaction, simplifying the process, looks something like this:
This equation represents solid copper (Cu (s)Cu (s)) and solid tin (Sn (s)Sn (s)) being heated and combined to form a solid bronze alloy (CuSn (s)CuSn (s)). In reality, the process involves the metals in their molten (liquid) state, and the resulting alloy’s composition can vary. After the metals are combined and stirred to ensure a uniform mixture, the molten bronze is poured into molds and allowed to cool and solidify into the desired shapes
| Property | Description |
|---|---|
| Color | Bronze has a characteristic golden to reddish-brown color, which can vary depending on the specific copper-to-tin ratio. |
| Density | It typically has a density around 7.4 to 8.9 grams per cubic centimeter, making it heavier than pure copper. |
| Melting Point | Bronze melts at approximately 1,742°F (950°C), which is lower than pure copper but varies with composition. |
| Hardness | It is harder than copper due to the presence of tin, making it resistant to wear and suitable for tools and machinery. |
| Conductivity | While bronze conducts electricity and heat, it does so less efficiently than copper, due to tin’s inclusion. |
| Malleability | Despite being hard, bronze is malleable and can be shaped into various forms, though it is less so than copper. |
| Corrosion Resistance | Bronze is highly resistant to corrosion, especially to seawater, making it ideal for maritime applications. |
| Patina Formation | Over time, bronze develops a green patina when exposed to air, which protects it from further corrosion. |
Bronze resists corrosion due to the formation of a protective oxide layer on its surface. This is especially effective against salt water, making bronze valuable for maritime uses.
Bronze develops a protective green or brown patina over time, which is a layer of copper carbonate. This occurs through the reaction:
2Cu + CO₂ + O₂ + H₂O → Cu₂(OH)₂CO₃
This layer shields the bronze from further corrosion.
Bronze shows low reactivity, making it stable and durable, especially in conditions promoting oxidation.
The thermal expansion of bronze is characterized by its expansion when heated and contraction when cooled, at rates determined by its specific composition
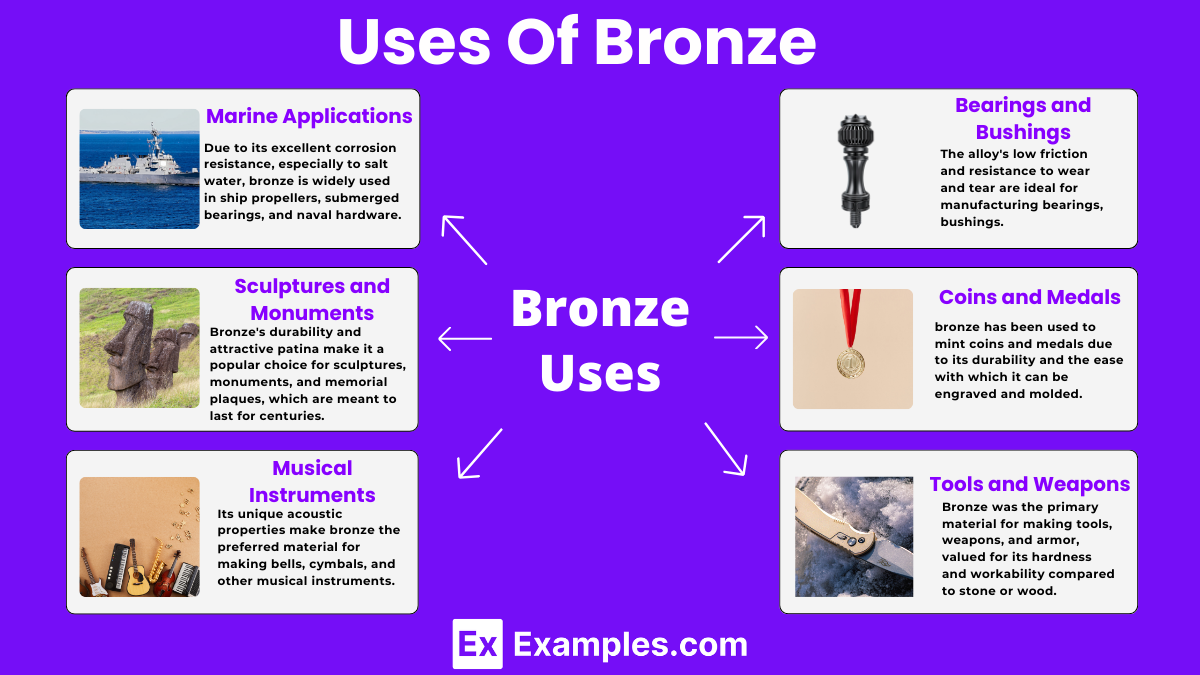
Due to its excellent corrosion resistance, especially to salt water, bronze is widely used in ship propellers, submerged bearings, and naval hardware.
Bronze’s durability and attractive patina make it a popular choice for sculptures, monuments, and memorial plaques, which are meant to last for centuries.
Its unique acoustic properties make bronze the preferred material for making bells, cymbals, and other musical instruments, providing a rich, resonant sound.
The alloy’s low friction and resistance to wear and tear are ideal for manufacturing bearings, bushings, and similar components in machinery and vehicles.
Historically, bronze has been used to mint coins and medals due to its durability and the ease with which it can be engraved and molded.
In ancient times, bronze was the primary material for making tools, weapons, and armor, valued for its hardness and workability compared to stone or wood.
Bronze is highly durable, able to withstand wear, pressure, and damage, making it suitable for long-lasting applications.
It exhibits excellent resistance to corrosion, especially against environmental conditions like salt water, reducing maintenance needs.
Bronze develops a beautiful patina over time, adding to its aesthetic appeal, which is especially valued in art and architecture.
Bronze surfaces have inherent antimicrobial properties, making them beneficial for reducing the spread of germs and bacteria.
The alloy can be easily cast into complex shapes and sizes, offering great versatility in manufacturing and artistic creation.
Bronze is favored for musical instruments due to its superior acoustic properties, producing a clear, resonant sound.
Bronze, an alloy of copper and tin, is prized for its strength, corrosion resistance, and beautiful patina, making it ideal for art and marine uses.
No, bronze is not just copper. It’s an alloy made primarily of copper and tin, which enhances its strength, durability, and corrosion resistance.
Bronze is still used but less commonly for tools and weapons due to modern materials like steel and aluminum, which are stronger and cheaper.
Bronze is expensive due to the cost of raw materials, copper and tin, and the process involved in alloying and casting it into usable forms.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Which civilization is known for starting the Bronze Age?
Egyptians
Mesopotamians
Greeks
Romans
What property of bronze makes it valuable for making sculptures?
High malleability
High melting point
Resistance to corrosion
Low density
Bronze is an alloy. What is an alloy?
A pure element
A mixture of two or more metals
A non-metallic compound
A gas
What is the melting point of bronze?
600°C
950°C
1085°C
1234°C
Which element is often added to bronze to improve its machinability?
Lead
Silver
Gold
Platinum
What color is bronze typically?
Silver
Gold
Reddish-brown
Blue
Which of the following is NOT a property of bronze?
High tensile strength
Low thermal conductivity
Low friction against other metals
Good electrical conductivity
Bronze was used to make which of the following ancient weapons?
Swords
Cannons
Rifles
Machine guns
What is the density of bronze?
5.0 g/cm³
7.0 g/cm³
8.7 g/cm³
9.2 g/cm³
Which modern application commonly uses bronze?
Electrical connectors
Airplane wings
Food containers
Fiber optics
Before you leave, take our quick quiz to enhance your learning!

