Potassium belongs to which group in the periodic table?
Group 1
Group 2
Group 3
Group 4

Butane, a term you might have heard in science class, is a fascinating member of the chemistry world, particularly known for its role in lighter fluid and portable stoves. At its core, Butane is a type of gas that belongs to the family of molecules known as hydrocarbons, compounds made up of hydrogen and carbon atoms. This gas is colorless, highly flammable, and exists in two forms, which differ slightly in their molecular structure but share the same chemical formula, C₄H₁₀. Understanding butane is not just about memorizing its formula; it’s about seeing its practical uses and its significance in everyday life, from fueling our camping trips to being a key ingredient in the production of various materials.
Butane, a colorless and highly flammable gas, is a type of hydrocarbon that belongs to the alkane family. With the chemical formula C₄H₁₀, it consists of four carbon atoms and ten hydrogen atoms. This gas is widely used in lighters, portable stoves, and as a fuel for some engines. Due to its ability to easily liquefy under pressure, butane is convenient for storage and transport in metal containers. Common in both natural gas and petroleum, butane plays a significant role in the fuel industry, providing a source of energy for heating and cooking in various applications.
| Property | Value |
|---|---|
| Formula | CH₃CH₂CH₂CH₃ |
| Hill Formula | C₄H₁₀ |
| Name | Butane |
| Alternate Names | Diethyl, Freon 600, Liquefied Petroleum Gas, n-Butane, R-600 |
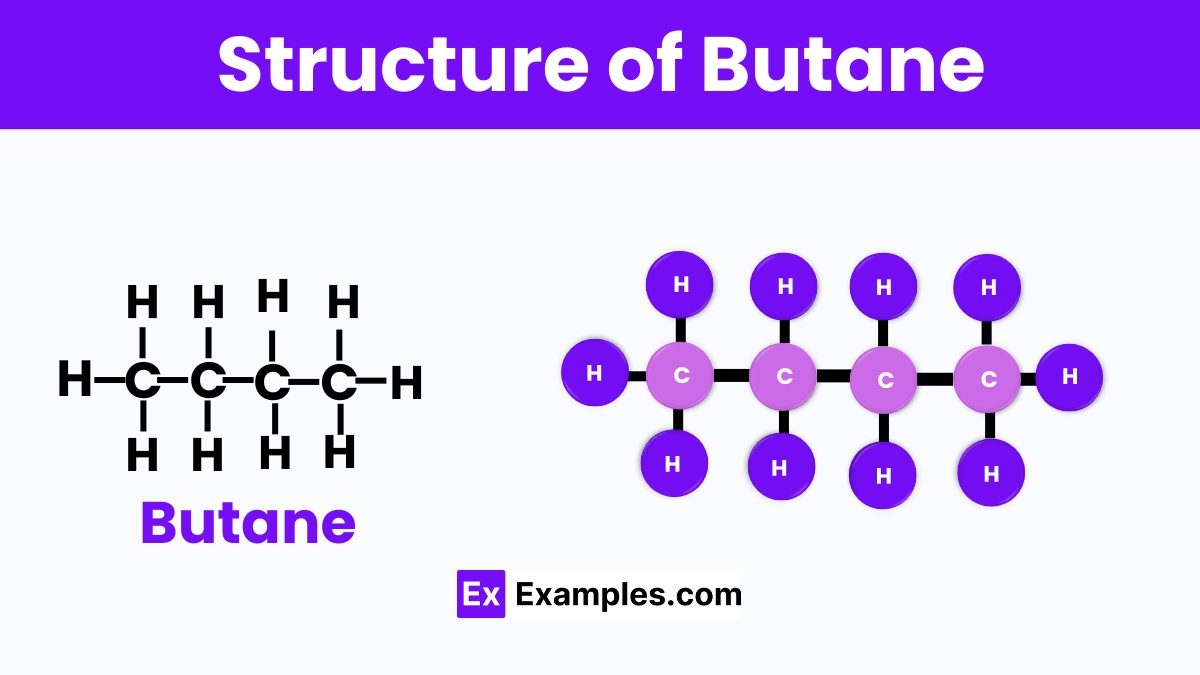
Butane is like a small chain made up of four carbon atoms linked together, with each carbon atom also holding onto hydrogen atoms to fill up its bonds. Picture it as a straight line of four circles (these are the carbon atoms) with smaller circles (the hydrogen atoms) attached around them. There are two versions of butane: one where this chain is straight, which we call n-butane, and another where the chain bends at the third carbon, creating a shape like a fork, known as isobutane. This simple structure makes butane a lightweight and versatile gas, widely used in everyday items like lighters and portable stoves.
Butane is created through a couple of interesting methods, each involving the transformation of molecules. One common way to prepare butane is through the distillation of crude oil in a refinery. During this process, the crude oil is heated, and its components are separated based on their boiling points. Butane boils at a relatively low temperature compared to other components, making it easy to separate. The chemical equation for this isn’t straightforward, as crude oil is a complex mixture, but think of it as heating a soup and capturing the steam that comes off first – that’s similar to how butane is collected.
Another method involves a chemical reaction called cracking. In cracking, larger hydrocarbon molecules are broken down into smaller ones at high temperatures. An example is the cracking of decane (a component of crude oil) into butane and hexene. The chemical equation for this process looks something like this:
This equation shows how a molecule of decane (C₁₀H₂₂) is transformed into a molecule of butane (C₄H₁₀) and a molecule of hexene (C₆H₁₂). Through these methods, butane is prepared and then used in various applications, from fueling your camping stove to filling up your lighter.
| Property | Description |
|---|---|
| State at Room Temperature | Butane is a gas at room temperature and atmospheric pressure. |
| Color | It is colorless, making it invisible to the eye in its pure form. |
| Odor | Pure butane is odorless, but an odorant is often added for safety reasons, so leaks can be detected. |
| Boiling Point | Butane boils at -0.5 degrees Celsius (31.1 degrees Fahrenheit), which means it turns into a liquid at slightly below freezing point of water. |
| Melting Point | Butane solidifies or freezes at about -138 degrees Celsius (-216 degrees Fahrenheit), which is way colder than any temperature experienced naturally on Earth’s surface. |
| Solubility in Water | Butane is not very soluble in water; it prefers to stay as a separate layer or float on top if mixed. |
| Density | It’s less dense than water, which is why it floats if you try to mix it with water. |
Butane is highly flammable, easily reacting with oxygen to ignite, creating carbon dioxide, water, and heat. This combustion process is crucial for its role as a fuel in various applications.
Equation: 2C₄H₁₀ + 13O₂ → 8CO₂ + 10H₂O + heat
Butane reacts with halogens (like chlorine or bromine) in a halogenation reaction. Under the influence of light and temperature, it replaces hydrogen atoms with halogen atoms.
Equation: C₄H₁₀ + Cl₂ → C₄H₉Cl + HCl
This results in the production of chlorobutane and hydrochloric acid.
Butane has the ability to transform into its isomers, rearranging its structure without altering its chemical formula. The conversion from n-butane to isobutane exemplifies its isomerization, highlighting its significance in creating various fuel types and chemicals.
Through the application of heat and sometimes catalysts, butane can undergo cracking, breaking down into smaller molecules. This process is instrumental in producing smaller hydrocarbons like ethylene and propylene, essential for plastic manufacturing.
Equation: C₄H₁₀ → C₂H₄ + C₂H₆
This shows butane’s capability to break down into ethylene (C₂H₄) and ethane (C₂H₆), illustrating its versatility in the chemical industry.
| Property | Value |
|---|---|
| CAS Registry Number | 106-97-8 |
| Beilstein Number | 969129 |
| PubChem Compound ID | 7843 |
| PubChem Substance ID | 24872900 |
| SMILES Identifier | CCCC |
| InChI Identifier | InChI=1/C4H10/c1-3-4-2/h3-4H2,1-2H3 |
| InChI Key | IJDNQMDRQITEOD-UHFFFAOYAE |
| RTECS Number | EJ4200000 |
| MDL Number | MFCD00009424 |
| Property | Value |
|---|---|
| NFPA Health Rating | 1 |
| NFPA Fire Rating | 4 |
| NFPA Reactivity Rating | 0 |
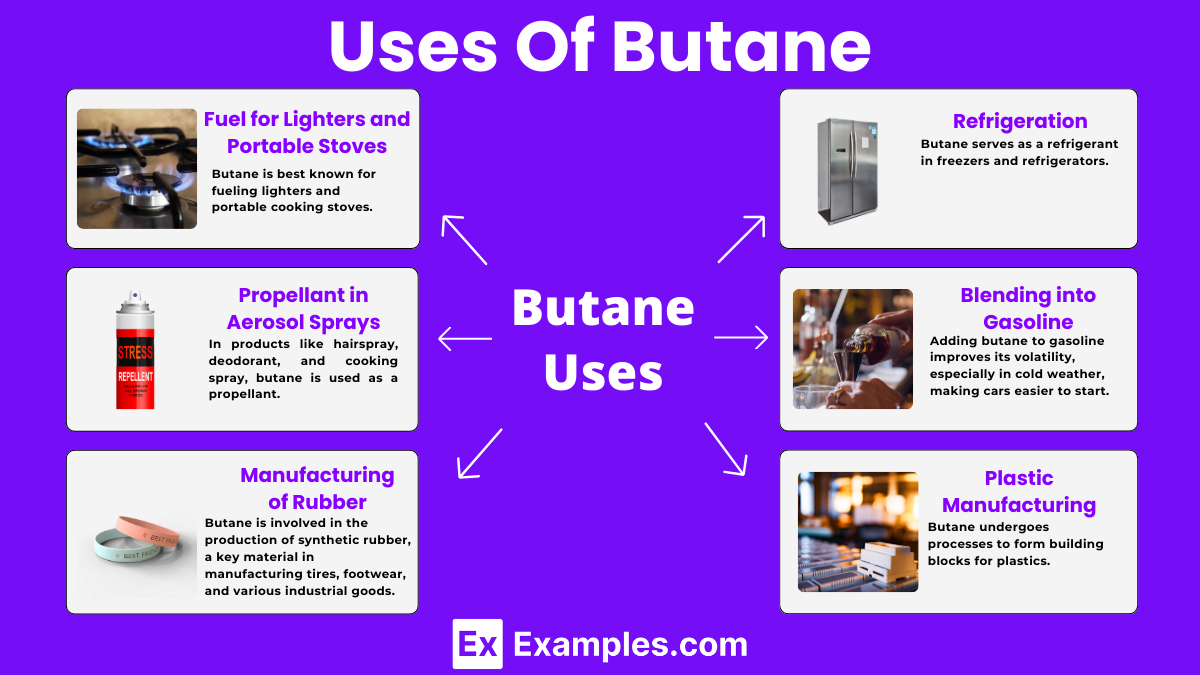
Butane is best known for fueling lighters and portable cooking stoves. Its ability to easily liquefy and vaporize makes it perfect for compact, portable devices that require a quick and reliable source of flame.
Butane serves as a refrigerant in freezers and refrigerators. Because it transitions from a liquid to a gas at a very low temperature, it’s efficient at removing heat, keeping our food cool and fresh.
In products like hairspray, deodorant, and cooking spray, butane is used as a propellant. It helps to push the product out of the can in a fine mist, making these products easy to use.
Butane is involved in the production of synthetic rubber, a key material in manufacturing tires, footwear, and various industrial goods. Its chemical properties facilitate the polymerization process needed to create rubber.
Adding butane to gasoline improves its volatility, especially in cold weather, making cars easier to start. This blending makes butane an essential component in automotive fuels.
Butane undergoes processes to form building blocks for plastics. By cracking butane, we get smaller molecules that are key ingredients in making plastics, providing materials for countless products we use daily.
Butane is the main component in lighter fluid for refillable lighters. Its high flammability and ease of handling make it the ideal choice for keeping your lighter ready to use.
In high concentrations, butane can be toxic, causing dizziness, unconsciousness, or even asphyxiation. Always use in well-ventilated areas to avoid inhalation risks.
Butane and propane are different hydrocarbons. Both are used as fuel but have varying boiling points, making them suitable for different applications.
Yes, butane is widely available for purchase. It’s commonly found in refillable lighters, portable stoves, and as a propellant in aerosol products.
Butane and propane pose similar health risks, including inhalation hazards. However, both are considered safe when used properly and in ventilated spaces.
When used correctly and in designed applications, butane is safe. It’s important to follow safety guidelines to prevent inhalation or fire hazards.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Potassium belongs to which group in the periodic table?
Group 1
Group 2
Group 3
Group 4
Which of the following best describes butane at room temperature?
Solid
Liquid
Gas
Plasma
What is the primary use of butane in households?
Lubricant
Fuel
Solvent
Antiseptic
Which element does not participate in forming potassium butane?
Carbon
Hydrogen
Oxygen
Potassium
What is the atomic number of potassium?
18
19
20
21
How many carbon atoms are present in butane?
2
3
4
5
Which type of bond is predominant in butane?
Ionic bond
Covalent bond
Metallic bond
Hydrogen bond
Potassium reacts vigorously with water to produce:
Potassium hydroxide and hydrogen gas
Potassium oxide and oxygen gas
Potassium chloride and chlorine gas
Potassium sulfate and sulfur dioxide
Butane belongs to which class of hydrocarbons?
Alkanes
Alkenes
Alkynes
Aromatics
What is the boiling point of butane?
-0.5°C
-1.0°C
-5.0°C
-0.5°F
Before you leave, take our quick quiz to enhance your learning!

