What is the atomic number of Cadmium?
46
48
50
52
Cadmium, a versatile element found in the earth’s crust, holds significant importance in various industries, from manufacturing batteries to crafting vibrant pigments. However, its environmental and health impacts cannot be overlooked. This comprehensive guide delves into the world of cadmium, exploring its uses, benefits, and the crucial measures needed to mitigate its risks. Whether you’re a scientist, an industry professional, or simply curious, our insights will illuminate the multifaceted role of cadmium in today’s world
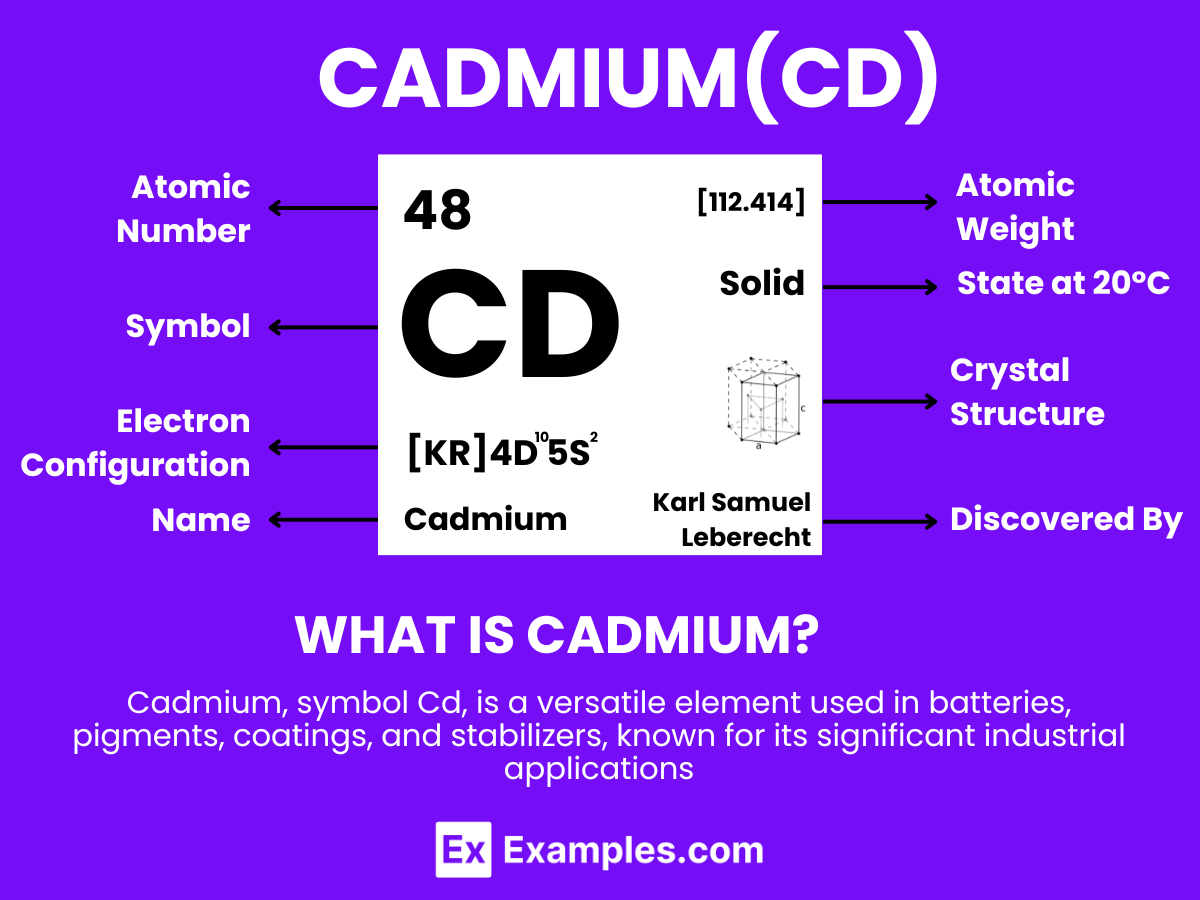
Cadmium is a soft, bluish-white metallic element that is malleable and known for its resistance to corrosion. With the atomic number 48. Cadmium is notable for its use and the environmental concerns associated with its toxicity. This element is not found as a free metal in nature but is usually obtained as a byproduct of zinc mining. Additionally, it is used in pigments, coatings, and plating to prevent corrosion. Cadmium’s properties also make it useful in the stabilization of plastics and as a neutron absorber in nuclear reactors. Despite its useful properties, the toxic nature of cadmium and its compounds necessitates careful handling and disposal to protect human health and the environment.
Formula: Cd
Composition: Consists of a single cadmium atom.
Bond Type: In its elemental form, cadmium does not form bonds as it is a pure element. Nonetheless, cadmium can engage in covalent or ionic bonding when it reacts with other elements.
Molecular Structure: As a pure element, cadmium does not have a molecular structure in the conventional sense, similar to compounds like H₂O. At room temperature, cadmium exists in a metallic state with a hexagonal close-packed crystalline structure.
Electron Sharing: In compounds, cadmium typically participates in electron sharing through covalent bonding or electron transfer via ionic bonding, depending on the characteristics of the other element(s) it bonds with.
Significance: Cadmium is known for its relatively low melting point (321°C or 610°F) compared to other metals and for its malleability. It is also recognized for its use in batteries, particularly nickel-cadmium batteries, and its distinctive yellow pigment used in paints.
Role in Chemistry: Cadmium has a critical role in various industrial applications, including electroplating to protect metals from corrosion. It is also used in the manufacturing of pigments, stabilizers for plastics, and as a neutron absorber in nuclear reactors. Despite its useful properties, cadmium is highly toxic, and its use is restricted in many applications to prevent environmental and health hazards.
Cadmium, with the chemical symbol Cd and atomic number 48, is a soft, bluish-white metal that is malleable and resistant to corrosion. As a member of the group 12 metals in the periodic table, cadmium shares some characteristics with its neighbors. Understanding the atomic structure of cadmium provides insights into its unique properties and applications. Here’s a detailed overview:
Cadmium, a soft, bluish-white metal, exhibits unique physical properties that make it suitable for various applications, including batteries, coatings, and pigments. Here is a detailed table outlining the essential physical properties of cadmium:
| Property | Value |
|---|---|
| Appearance | Bluish-white, lustrous metal |
| Atomic Number | 48 |
| Atomic Weight | 112.414 u |
| Melting Point | 321.07°C (609.93°F) |
| Boiling Point | 767°C (1413°F) |
| Density | 8.65 g/cm³ at 20°C |
| State at Room Temperature | Solid |
| Electrical Conductivity | 13.3 × 10⁶ S/m |
| Thermal Conductivity | 96.6 W/(m·K) |
| Heat Capacity | 0.230 J/g·K |
| Crystal Structure | Hexagonal close-packed (hcp) |
2Cd+O₂ →2CdO
Cadmium reacts with oxygen to form cadmium oxide.
Cadmium does not react directly with water under normal conditions, so no equation is provided for this reaction.
Cadmium’s reaction with bases like sodium hydroxide (NaOH) to form cadmium hydroxide can be represented as:
CdO+2NaOH→Cd(OH)₂ +2Na+
Cd+Cl₂ →CdCl₂
CdCO₃→CdO+CO₂ ↑
| Property | Value |
|---|---|
| Melting Point | 321°C (610°F) |
| Boiling Point | 767°C (1413°F) |
| Heat of Fusion | 6.21 kJ/mol |
| Heat of Vaporization | 99.87 kJ/mol |
| Specific Heat Capacity | 230 J/(kg·K) |
| Thermal Conductivity | 96.6 W/(m·K) |
| Property | Value |
|---|---|
| Atomic Number | 48 |
| Atomic Weight | 112.414 u |
| Density | 8.65 g/cm³ at 20°C |
| Crystal Structure | Hexagonal Close-packed (hcp) |
| Young’s Modulus | 50 GPa |
| Poisson’s Ratio | 0.30 |
| Hardness (Mohs) | Soft (approx. 2) |
| Property | Value |
|---|---|
| Electrical Conductivity | 13.3 × 10⁶ S/m |
| Electrical Resistivity | 7.2 × 10⁻⁸ Ω·m (at 20°C) |
| Magnetic Susceptibility | -19.8 × 10⁻⁶ cm³/mol (at 293 K) |
| Property | Value |
|---|---|
| Natural Isotopes | Cd-106, Cd-108, Cd-110, Cd-111, Cd-112, Cd-113, Cd-114, Cd-116 |
| Half-life of Cd-113 | Stable |
| Neutron Cross Section | 2520 barns (for Cd-113) |
| Isotope Abundance | Varies by isotope |
A semiconductor useful in photoresistors and solar cells.
Key in photovoltaic cells, offering significant photoconductivity.
Utilized in electroplating and as a catalyst.
Applied in electroplating and as an electrolyte.
Used in pyrotechnics and as a colorant.
Employed in the production of cadmium pigments and as a stabilizer.
The preparation of cadmium involves extracting it from zinc ores, where it is commonly found as a byproduct. The process is complex, involving several steps to isolate cadmium from other elements present in the ore. Here’s an overview of the key stages in the preparation of cadmium:
1. Mining and Crushing: The initial step involves mining zinc ores and crushing them into smaller pieces to facilitate further processing.
2. Concentration: The crushed ore undergoes concentration processes, such as flotation, to increase the zinc content while removing other materials.
3. Roasting: The concentrated ore is then roasted in the presence of oxygen. This step converts the zinc and cadmium sulfides present in the ore to their respective oxides.
4. Leaching: The roasted ore is leached with sulfuric acid, which dissolves the zinc and cadmium oxides, separating them from the rest of the materials.
5. Electrolysis: Zinc is primarily extracted from the solution through electrolysis. Cadmium is usually found as an impurity in the zinc and is collected from the electrolyte or as a precipitate.
6. Distillation: The cadmium obtained from the previous step often contains impurities. To purify cadmium, it is distilled in a vacuum. During this process, cadmium is vaporized and then condensed into a pure form, as it has a lower boiling point than most impurities
Cadmium has several isotopes, each with unique properties and stability. The table below lists some of the most significant isotopes of cadmium, highlighting their abundance and applications.
| Isotope | Natural Abundance | Half-life | Applications |
|---|---|---|---|
| Cd-106 | 1.25% | Stable | Low-energy gamma detection, geochronology |
| Cd-108 | 0.89% | Stable | – |
| Cd-110 | 12.49% | Stable | – |
| Cd-111 | 12.80% | Stable | – |
| Cd-112 | 24.13% | Stable | – |
| Cd-113 | 12.22% | 7.7×10¹⁵ years | Neutron capture studies, cadmium telluride detectors |
| Cd-114 | 28.73% | Stable | – |
| Cd-116 | 7.49% | 2.8×10¹⁹ years | Low-level gamma-ray detectors |
Cadmium plays a pivotal role in various applications across multiple industries, leveraging its unique physical and chemical properties. Below are some of the key uses of cadmium:
The production of Cadmium primarily occurs as a byproduct of the mining, smelting, and refining of zinc and, to a lesser extent, lead and copper. The process involves several key steps to extract Cadmium from these base metals:
This process not only yields Cadmium but also helps in the efficient utilization of zinc ores, making it an essential part of the non-ferrous metal industry.
Cadmium’s unique properties have led to its use in a variety of applications across several industries:
cadmium, a versatile metal with a hexagonal close-packed structure, exhibits unique thermodynamic, material, electromagnetic, and nuclear properties. Despite its utility in various industrial applications, including batteries and pigments, the toxicity of cadmium necessitates careful handling and regulation. Its significance in technology and potential hazards highlight the importance of understanding cadmium’s comprehensive properties.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Cadmium?
46
48
50
52
What is the primary source of Cadmium in nature?
Hematite
Bauxite
Sphalerite
Chalcopyrite
Which of the following is a common use of Cadmium?
Construction material
Rechargeable batteries
Food additive
Textile dye
What is the color of Cadmium in its pure form?
Gray
Yellow
Blue
Silver
Which property of Cadmium makes it useful in corrosion-resistant coatings?
High melting point
High reactivity
Good electrical conductivity
Resistance to oxidation
Cadmium is classified as which type of element?
Alkali metal
Alkaline earth metal
Transition metal
Post-transition metal
Which of the following is a health risk associated with Cadmium exposure?
Vitamin deficiency
Kidney damage
Hair loss
Increased appetite
In which industry is Cadmium used to stabilize PVC?
Construction
Textile
Plastics
Food processing
What is the melting point of Cadmium?
234°C
321°C
419°C
612°C
Which of the following Cadmium compounds is used in pigments?
Cadmium sulfide
Cadmium oxide
Cadmium chloride
Cadmium nitrate
Before you leave, take our quick quiz to enhance your learning!

