What is the chemical formula of calcium carbonate?
CaCO₃
CaO
Ca(OH)₂
CaCl₂

Calcium carbonate (CaCO₃), a ionic compound formed through ionic bonding between calcium cations (Ca²⁺) and carbonate anions (CO₃²⁻), is widely encountered in various geological and biological contexts. It is a key component of materials such as limestone, chalk, and marble, which are extensively used in construction and sculpture. In the realm of chemistry, calcium carbonate is a vital substance utilized in various applications due to its stability and versatility. Additionally, it plays a crucial role in biology, serving as the material for the shells of marine organisms, snails, and eggs, as well as the skeletons of coral reefs.
| Property | Value |
|---|---|
| Formula | CaCO₃ |
| Hill Formula | CCaO₃ |
| Name | Calcium carbonate |
| Alternate Names | Calcite, Chalk, Limestone, Marble, Pearl, Aeromatt |
| Common Name | Description |
|---|---|
| Limestone | Sedimentary rock used in building and as a primary ingredient in cement production. |
| Chalk | Soft, white, porous form used for drawing, classroom chalk, and in climbing. |
| Marble | Metamorphic rock formed from limestone, used in architecture and sculpture. |
| Calcite | The most stable form of calcium carbonate, occurring naturally or as a ground powder in various industries. |
| Aragonite | A naturally occurring polymorph of calcium carbonate, found in marine environments and used in aquariums. |
| Lime | Refers to products derived from limestone, such as quicklime and hydrated lime, used in construction and industrial processes. |
| Precipitated Calcium Carbonate (PCC) | Manufactured calcium carbonate produced under controlled conditions, used in the production of paper, paint, and plastics. |
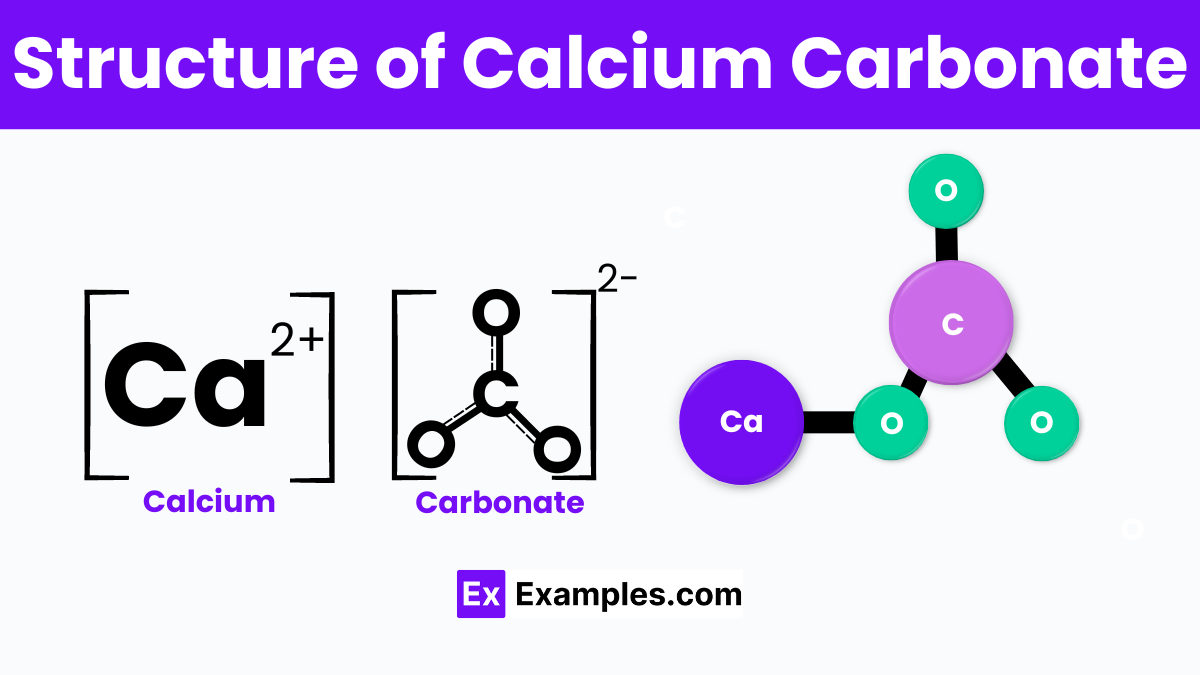
Calcium carbonate is characterized by its unique chemical structure, represented by the formula CaCO₃. At its core, this compound consists of one calcium (Ca) ion bonded to one carbonate (CO₃) ion. The carbonate ion is a polyatomic ion with a trigonal planar geometry, comprising one carbon atom centrally bonded to three oxygen atoms in a symmetrical arrangement. This geometry allows for the formation of stable, solid structures, contributing to calcium carbonate’s common occurrence in nature as minerals such as calcite, aragonite, and vaterite. These forms differ in their crystallography: calcite forms a trigonal crystal system, aragonite in an orthorhombic system, and vaterite is hexagonal. This structural diversity allows calcium carbonate to manifest in various physical forms, from the hard shells of marine organisms to the stalactites and stalagmites found in caves, showcasing its vast presence and significance in the natural world.
Calcium carbonate, a widely used compound, can be prepared using several straightforward methods. One common approach is the carbonation process, where calcium hydroxide reacts with carbon dioxide gas to form calcium carbonate and water. This reaction can be summarized by the equation:
This method not only occurs naturally, such as in limestone caves leading to stalactite and stalagmite formation, but is also utilized industrially to produce calcium carbonate for various uses.
Another method is the precipitation technique, which involves mixing solutions that contain calcium ions and carbonate ions. When these mix, calcium carbonate precipitates, or settles out of the solution as a solid, as shown by the equation:
This approach is particularly useful for producing calcium carbonate with specific particle sizes and shapes, catering to specialized applications.
Additionally, calcium carbonate can be directly obtained from natural sources like limestone, marble, and chalk, through processes that involve quarrying, crushing, and grinding these materials. Lastly, some organisms naturally produce calcium carbonate for their shells and skeletons, a process known as biogenic formation. Each of these methods provides a unique way to produce calcium carbonate, meeting the diverse demands across different industries.
| Property | Description |
|---|---|
| Appearance | White powder or colorless crystals |
| Molecular Weight | 100.0869 g/mol |
| Density | 2.71 g/cm³ (calcite), 2.93 g/cm³(aragonite) |
| Melting Point | Decomposes at 825°C (1517°F) without melting |
| Solubility in Water | Sparingly soluble (0.013 g/L at 25°C for calcite) |
| Crystal Structure | Trigonal (calcite), Orthorhombic (aragonite) |
| Hardness (Mohs) | 3 (calcite), 3.5-4 (aragonite) |
| Refractive Index | 1.49 (calcite), 1.53 (aragonite) |
| pH | Alkaline (basic) |
Calcium carbonate has a molecular weight of 100.0869 g/mol, which is the sum of the atomic weights of one calcium atom, one carbon atom, and three oxygen atoms.
It is poorly soluble in pure water, with a solubility of approximately 0.013 g/L at 25°C. However, its solubility increases in the presence of carbon dioxide due to the formation of soluble calcium bicarbonate.
Being a base, calcium carbonate has an alkaline reaction. When dissolved in water, it can increase the pH of the solution due to the release of carbonate ions, which can combine with hydrogen ions.
Calcium carbonate reacts with acids to produce carbon dioxide gas, water, and a salt. For example, when it reacts with hydrochloric acid, calcium chloride, water, and carbon dioxide are formed.
Equation: CaCO₃+2HCl→CaCl₂+H2O+CO₂CaCO₃+2HCl→CaCl₂+H₂O+CO₂.
It can form complexes with ions in water, contributing to the hardness of water. The calcium ions released from calcium carbonate can bind with other anions in water, such as sulfate or carbonate, forming compounds that precipitate out of solution.
Calcium carbonate is stable under normal conditions but can react with stronger acids, releasing carbon dioxide. It is also sensitive to acid rain, which can lead to the dissolution of calcium carbonate-based materials over time.
When heated above 840°C, calcium carbonate decomposes into calcium oxide (lime) and carbon dioxide gas, a reaction used in the lime-making process.
Equation: CaCO₃→CaO+CO₂CaCO₃→CaO+CO₂.
| Identification | Value |
|---|---|
| CAS Registry Number | 471-34-1 |
| Beilstein Number | 8008338 |
| PubChem Compound ID | 10112 |
| SMILES Identifier | C(=O)([O-])[O-].[Ca+2] |
| InChI Identifier | InChI=1/CH2O3.Ca/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2/fCO3.Ca/q-2;m |
| EU Number | 215-279-6 |
| Gmelin Number | 8544 |
| RTECS Number | FF9335000 |
| MDL Number | MFCD00010906 |
Calcium carbonate is beneficial for bone health and treating indigestion, but excessive intake can cause side effects like kidney stones.
Yes, Tums is a brand of antacid that primarily contains calcium carbonate, used to relieve heartburn and indigestion.
No, calcium carbonate is a mineral used for calcium supplementation, whereas vitamin D helps the body absorb calcium but is not a mineral.
Taking large doses of calcium and vitamin D together can lead to excessive calcium in the blood, potentially causing harmful side effects.
Yes, taking Vitamin D3 and calcium carbonate together is common and beneficial, as Vitamin D3 enhances calcium absorption from the gastrointestinal tract.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical formula of calcium carbonate?
CaCO₃
CaO
Ca(OH)₂
CaCl₂
Calcium carbonate is commonly found in which natural material?
Granite
Limestone
Basalt
Sandstone
Which of the following is a use of calcium carbonate?
Fuel
Antacid
Plasticizer
Fertilizer
Calcium carbonate decomposes upon heating to form which compounds?
Calcium oxide and carbon dioxide
Calcium hydroxide and water
Calcium sulfate and sulfur dioxide
Calcium chloride and chlorine
What is the common name for the mineral form of calcium carbonate?
Quartz
Gypsum
Calcite
Hematite
Calcium carbonate reacts with hydrochloric acid to produce:
Calcium chloride, water, and carbon dioxide
Calcium hydroxide and chlorine gas
Calcium sulfate and water
Calcium nitrate and nitrogen gas
Which property of calcium carbonate makes it useful in agriculture?
High solubility in water
Acidity
Alkalinity
Volatility
Which of the following is a polymorph of calcium carbonate?
Aragonite
Dolomite
Anhydrite
Siderite
In which industry is calcium carbonate commonly used as a filler?
Textile
Plastic
Metal
Ceramic
Which reaction demonstrates the role of calcium carbonate in the carbon cycle?
Photosynthesis
Combustion
Precipitation
Decomposition
Before you leave, take our quick quiz to enhance your learning!

