What is the chemical formula of Calcium Hydroxide?
CaH₂
CaOH
Ca(OH)₂
CaO₂H

Calcium hydroxide is also known as slaked lime and it is a compound with the chemical formula Ca(OH)₂. It is commonly found in both laboratories and everyday products such as mortar and plaster. In chemistry, it is categorized as a base because it reacts with acids to form salts and water. This white powdery substance is highly versatile, used in water purification, food preparation, and even in the dental field to maintain healthy teeth. Its ability to adjust acidity makes it invaluable in various industrial and environmental applications, showcasing the practical uses of bases in our daily lives.
| Property | Value |
|---|---|
| Formula | Ca(OH)₂ |
| Hill Formula | H₂CaO₂ |
| Name | Calcium hydroxide |
| IUPAC Name | Calcium dihydroxide |
| Alternate Names | Calcium dihydroxide, Calcium hydrate, Hydrated lime, Slaked lime |
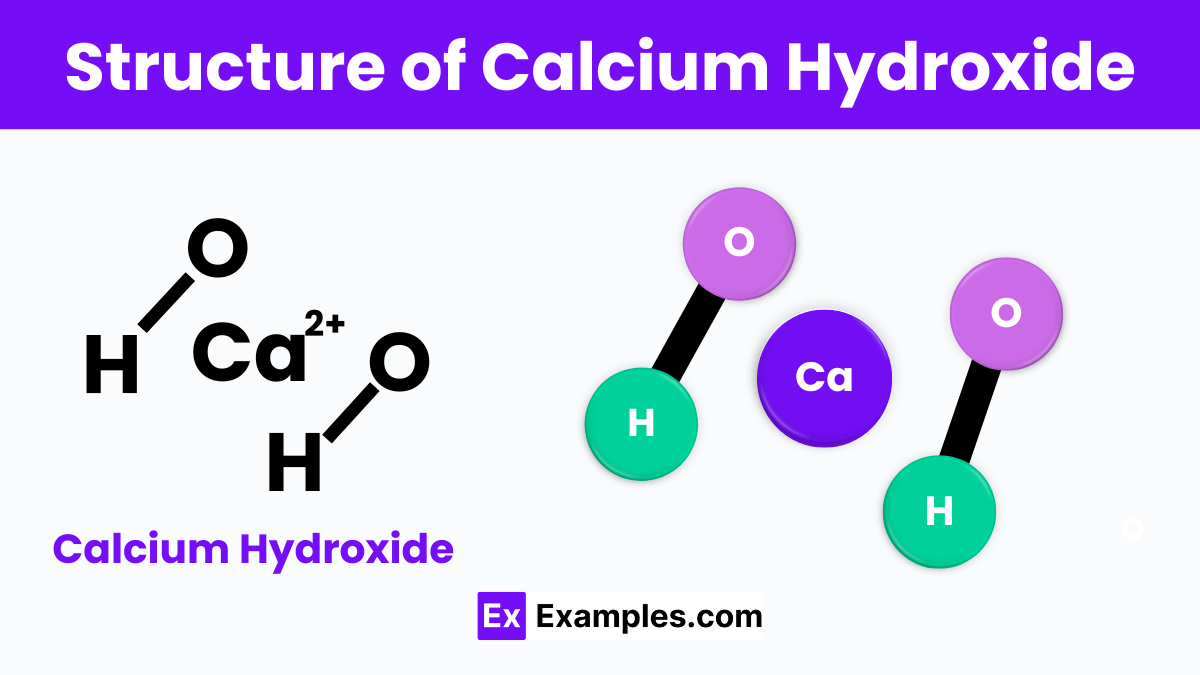
Calcium hydroxide is composed of one calcium atom bonded to two hydroxide groups. The chemical structure can be represented as Ca(OH)₂. In this structure, the calcium atom is in the center, linked to two hydroxide ions, each consisting of one oxygen and one hydrogen atom. These bonds form a layered crystalline structure that is slightly soluble in water. This arrangement allows calcium hydroxide to readily react with carbon dioxide in the air to form calcium carbonate, a process important in many environmental and industrial applications.
Calcium hydroxide is prepared by adding water to calcium oxide, a process known as slaking. The chemical reaction involved is quite straightforward and can be represented by the equation:
In this reaction, calcium oxide (CaO), commonly called quicklime, reacts vigorously with water (H₂O), producing calcium hydroxide (Ca(OH)₂) and releasing a significant amount of heat. This exothermic reaction results in a fine dry powder of calcium hydroxide, often referred to as slaked lime. This substance is used in various applications, such as in construction for making plasters and mortars and in agriculture to neutralize acidic soils.
| Property | Description |
|---|---|
| Appearance | White powder or colorless crystals |
| Odor | Odorless |
| Formula | Ca(OH)₂ |
| Density | 2.211 g/cm³ (solid) |
| Melting Point | Approximately 580°C (decomposes) |
| Solubility | Slightly soluble in water; solubility decreases as temperature increases |
| pH | Highly basic (about 12.4 when in a saturated solution) |
| Property | Value |
|---|---|
| CAS Registry Number | 1305-62-0 |
| PubChem Compound ID | 14777 |
| PubChem Substance ID | 24868536 |
| SMILES Identifier | [OH-].[OH-].[Ca+2] |
| InChI Identifier | InChI=1/Ca.2H2O/h;21H2/q+2;;/p-2/fCa.2HO/h;21h/qm;2*-1 |
| RTECS Number | EW2800000 |
| MDL Number | MFCD00010901 |
| Property | Value |
|---|---|
| NFPA Health Rating | 3 |
| NFPA Fire Rating | 0 |
| NFPA Reactivity Rating | 0 |
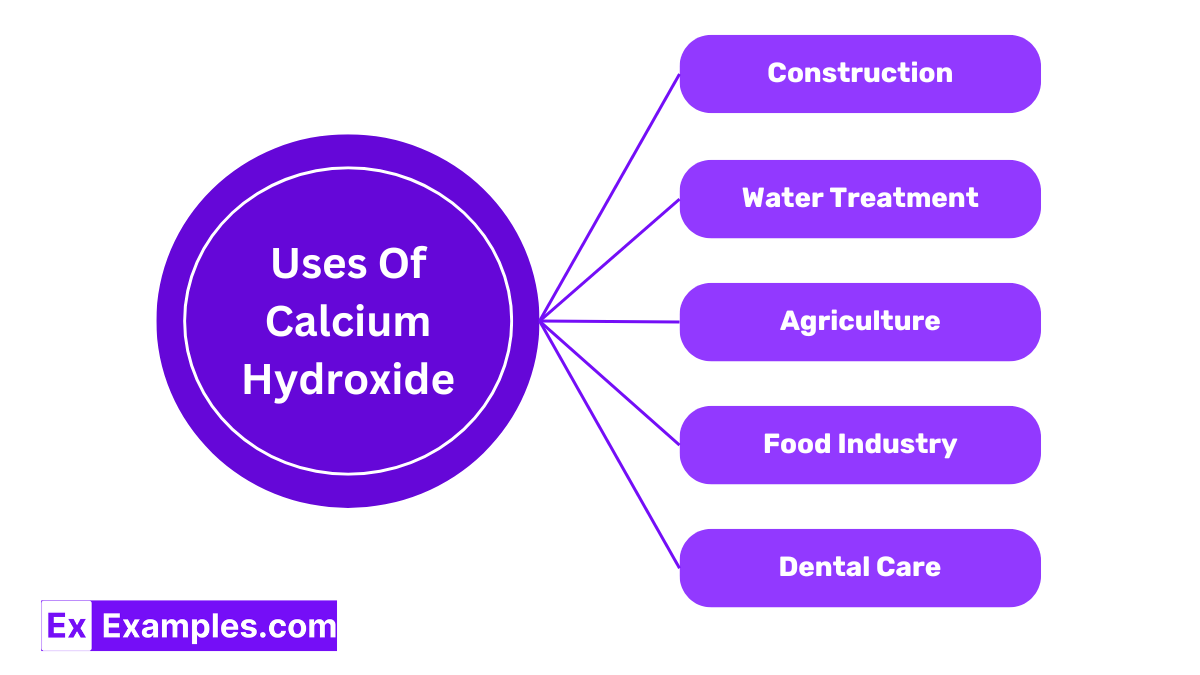
Calcium hydroxide is widely used in the construction industry for making mortar and plaster. It helps improve the workability of mortar and increases its durability once set. It also aids in the curing process by reacting with carbon dioxide in the air to form a hard, durable surface layer of calcium carbonate.
It plays a crucial role in water treatment processes. By adjusting the pH of water, calcium hydroxide helps in purifying drinking water and treating wastewater. It is also used to remove impurities by precipitating unwanted ions through the formation of insoluble compounds.
Farmers use calcium hydroxide to adjust soil acidity, an essential step in improving soil health and crop yields. It neutralizes acidic soils, allowing plants to better absorb nutrients.
This compound is used as a food additive known as E526. It is used in the processing of various foods, such as corn tortillas and pickles, to improve texture and maintain freshness. Additionally, it is used in sugar refining to purify raw sugar and in the clarification of juices.
Calcium hydroxide is used in dentistry for its antibacterial properties. It is a common ingredient in root canal treatments, helping to reduce infections and promote the formation of secondary tooth material.
Calcium hydroxide is a strong base, commonly known as slaked lime, with a formula of Ca(OH)₂.
Calcium hydroxide has a high pH, typically around 12.4, indicating strong basicity when dissolved in water.
Calcium hydroxide can cause skin irritation and burns; it is not considered safe for direct skin contact.
While not highly toxic, calcium hydroxide can be harmful if inhaled or contacted with skin and eyes, requiring careful handling.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical formula of Calcium Hydroxide?
CaH₂
CaOH
Ca(OH)₂
CaO₂H
What is the common name for Calcium Hydroxide?
Limewater
Slaked lime
Baking soda
Quicklime
What is the primary use of Calcium Hydroxide in the construction industry?
Insulation
Cement additive
Drywall production
Paint pigment
Which property of Calcium Hydroxide makes it useful in water treatment?
High solubility
Basicity
Acidity
Oxidizing agent
What happens when Calcium Hydroxide is dissolved in water?
It forms an acidic solution
It decomposes
It remains insoluble
It forms a basic solution
Which form of Calcium Hydroxide is used to detect carbon dioxide in the laboratory?
Hydrated lime
Slaked lime powder
Limewater
Anhydrous calcium hydroxide
What is the solubility of Calcium Hydroxide in water at 25°C?
Highly soluble
Moderately soluble
Sparingly soluble
Insoluble
What is the pH of a saturated solution of Calcium Hydroxide?
Around 3
Around 5
Around 7
Around 12
What is the color of pure Calcium Hydroxide?
White
Gray
Yellow
Brown
In which process is Calcium Hydroxide used to remove impurities?
Water treatment
Metal refining
Paper manufacturing
Food preservation
Before you leave, take our quick quiz to enhance your learning!

