What is the shape of the carbon dioxide molecule?
Linear
Bent
Trigonal planar
Tetrahedral

Carbon dioxide is a gas that you can’t see or smell, but it’s all around us. When we breathe out, we release CO₂ into the air. It’s also made when things like cars and factories burn fuel. Trees and plants need Carbon dioxide to grow, taking it from the air and giving us oxygen in return. But, too much Carbon dioxide in the air can cause problems for our planet, like making the Earth warmer than it should be. This gas plays a big role in our world, from helping plants grow to affecting our climate.
Carbon dioxide, often abbreviated as CO₂, is a covalent compound made of carbon and oxygen. In simpler terms, it’s a kind of gas that forms when carbon atoms bond closely with two oxygen atoms. This bonding happens in a special way called covalent bonding, where the atoms share electrons with each other. You can’t see or smell carbon dioxide, but it’s a big part of the air around us and plays a crucial role in life on Earth. Plants use CO₂ to make food through a process called photosynthesis, turning CO₂ and sunlight into energy. However, too much CO₂ in the atmosphere can lead to climate change, making it an important substance to understand in chemistry and environmental science.
| Property | Value |
|---|---|
| Formula | CO₂ |
| Name | Carbon Dioxide |
| Alternate Names | Anhydride Carbonique, Carbon Dioxide, Compressed, Carbonic Anhydride, Freon 744 R-744 |
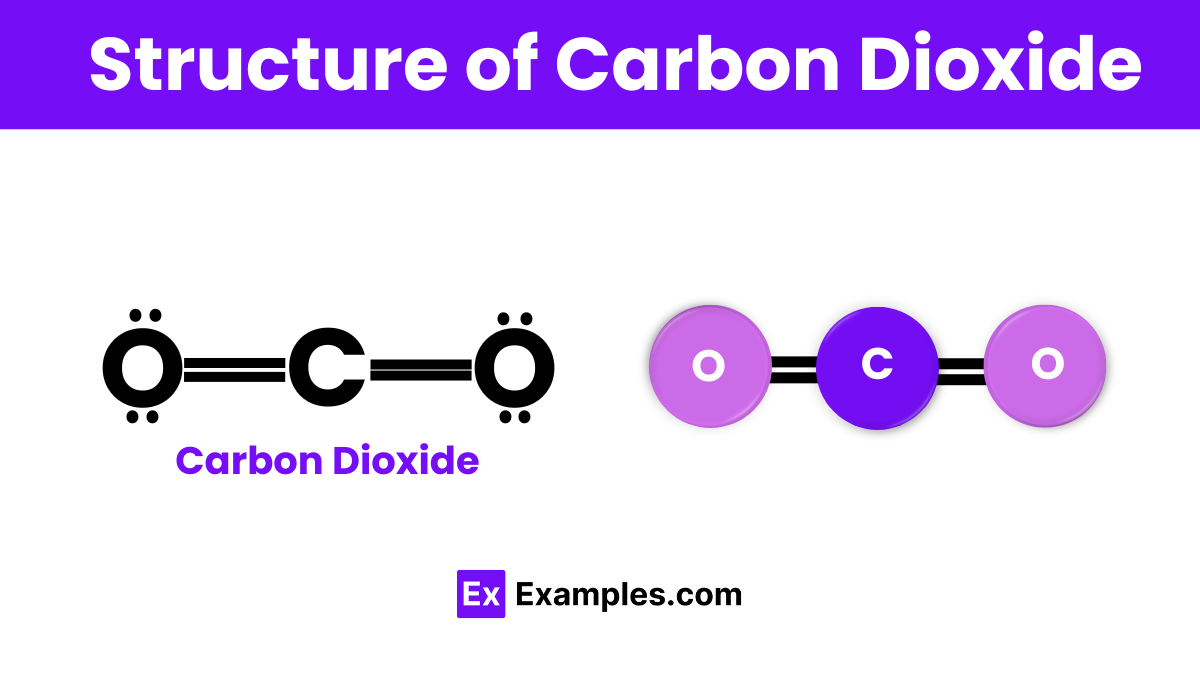
The structure of carbon dioxide (CO₂) is straightforward yet fascinating. At its core, CO₂ consists of one carbon atom that forms double bonds with two oxygen atoms. This configuration gives CO₂ a linear shape, meaning all three atoms lie in a straight line. The double bonds between the carbon and oxygen atoms are strong and stable, making CO₂ a non-polar molecule. This structural simplicity allows CO₂ to be a gas at room temperature, easily mixing with the air around us. Its linear, symmetrical form is key to its behavior and interactions in the environment and industrial processes.
Carbon dioxide (CO₂) can be prepared through several methods, one of the most common being the chemical reaction between a carbonate or bicarbonate and an acid. For example, when calcium carbonate (CaCO₃), commonly found in chalk or limestone, reacts with hydrochloric acid (HCl), carbon dioxide gas is released as a byproduct. The equation for this reaction is:
Another way to produce CO₂ is by heating carbonate compounds. When magnesium carbonate (MgCO₃) is heated, it decomposes to form magnesium oxide (MgO) and releases carbon dioxide gas. The equation for this process is:
These reactions not only illustrate how CO₂ can be generated in a laboratory setting but also reflect the compound’s involvement in natural geological processes and its role in the carbon cycle.
| Property | Description |
|---|---|
| Molecular Weight | 44.01 g/mol |
| Phase at Room Temperature | Gas |
| Color | Colorless |
| Odor | Odorless |
| Density | 1.977 kg/m³ (at 0°C and 1 atm) |
| Melting Point | -56.6°C (sublimes) |
| Boiling Point | -78.5°C (at 1 atm, sublimation point) |
| Solubility in Water | 1.45 g/L (at 20°C and 1 atm) |
| Critical Temperature | 31.1°C |
| Critical Pressure | 7.38 MPa |
Carbon dioxide dissolves in water to form carbonic acid (H₂CO₃), albeit weakly, affecting the water’s pH level. This reaction is crucial for the carbon cycle and aquatic life balance. CO₂+H₂O→H₂CO₃
CO₂ reacts with bases (alkalis) to produce carbonates or bicarbonates. For example, when CO₂ is passed through a solution of sodium hydroxide (NaOH), sodium carbonate (Na₂CO₃) is formed.
CO₂+2NaOH→Na₂CO₃+H2O
In the presence of water and calcium ions, CO₂ can lead to the formation of calcium carbonate (CaCO₃), a key component of limestone, through a series of reactions. This process is vital in geological formations and the ocean’s carbon cycle.
Carbon dioxide is a key reactant in the photosynthesis process by which plants convert CO₂ and water into glucose and oxygen, using sunlight as energy. This reaction is fundamental to life on Earth.
6CO₂+6H₂O→C₆H₁₂O₆+6O₂
At temperatures below its sublimation point (-78.5°C), solid CO₂ (dry ice) converts directly into CO₂ gas without becoming liquid. This property makes dry ice useful for refrigeration and creating fog effects.
| Property | Value |
|---|---|
| CAS Registry Number | 124-38-9 |
| Beilstein Number | 1900390 |
| PubChem Compound ID | 280 |
| PubChem Substance ID | 24857758 |
| SMILES Identifier | C(=O)=O |
| InChI Identifier | InChI=1/CO2/c2-1-3 |
| RTECS Number | FF6400000 |
| MDL Number | MFCD00011491 |
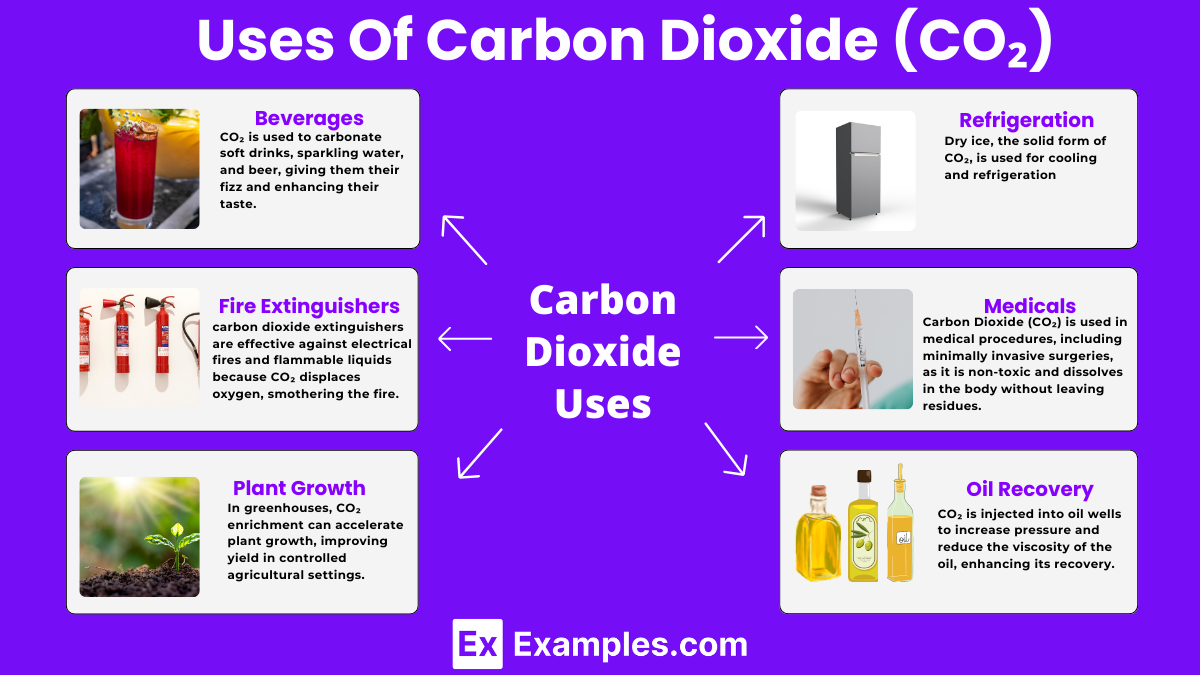
CO₂ is used to carbonate soft drinks, sparkling water, and beer, giving them their fizz and enhancing their taste.
Carbon dioxide extinguishers are effective against electrical fires and flammable liquids because CO₂ displaces oxygen, smothering the fire.
In greenhouses, CO₂ enrichment can accelerate plant growth, improving yield in controlled agricultural settings.
Dry ice, the solid form of CO₂, is used for cooling and refrigeration. It’s particularly useful for transporting perishable goods and creating fog effects in entertainment.
Carbon Dioxide (CO₂) is used in medical procedures, including minimally invasive surgeries, as it is non-toxic and dissolves in the body without leaving residues.
Scientists use CO₂ in photosynthesis studies to understand plant growth and improve crop production strategies.
CO₂ is injected into oil wells to increase pressure and reduce the viscosity of the oil, enhancing its recovery.
Carbon dioxide is used to cure concrete, speeding up the hardening process and potentially trapping CO₂ permanently in buildings and structures.
Excess carbon dioxide can lead to health issues like headaches, dizziness, breathing difficulties, and an increased heart rate, affecting overall well-being.
Normal CO₂ levels in a person range between 35-45 mmHg, indicating healthy respiratory and metabolic function.
Carbon dioxide is not flammable. It is used in fire extinguishers because it suffocates flames by displacing oxygen.
Current CO₂ levels in the air average about 417 ppm, a significant increase from pre-industrial levels, influencing global climate patterns.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the shape of the carbon dioxide molecule?
Linear
Bent
Trigonal planar
Tetrahedral
Which chemical property of carbon dioxide contributes to ocean acidification?
Reactivity with water
Solubility in water
Low boiling point
High density
How is carbon dioxide typically removed from the atmosphere by natural processes?
Combustion
Photosynthesis
Decomposition
Evaporation
What is the common use of carbon dioxide in the food industry?
Flavoring agent
Preservative
Leavening agent
Carbonation of beverages
What is the state of carbon dioxide at room temperature and pressure?
Solid
Liquid
Gas
Plasma
What is the main component of dry ice?
Frozen water
Solid nitrogen
Solid carbon dioxide
Frozen methane
Which of the following industries is a major emitter of carbon dioxide?
Agriculture
Textile
Transportation
Electronics
How does carbon dioxide contribute to the greenhouse effect?
Reflecting sunlight
Trapping heat in the atmosphere
Absorbing UV radiation
Reducing oxygen levels
What is the pH of a carbon dioxide solution in water?
Neutral (7)
Slightly acidic (5-6)
Strongly acidic (2-3)
Alkaline (8-9)
Which gas is the primary product of the combustion of carbon-based fuels?
Methane
Oxygen
Nitrogen
Carbon dioxide
Before you leave, take our quick quiz to enhance your learning!

