What is the primary function of catalase in the cell?
DNA replication
Protein synthesis
Breakdown of hydrogen peroxide
Glucose metabolism

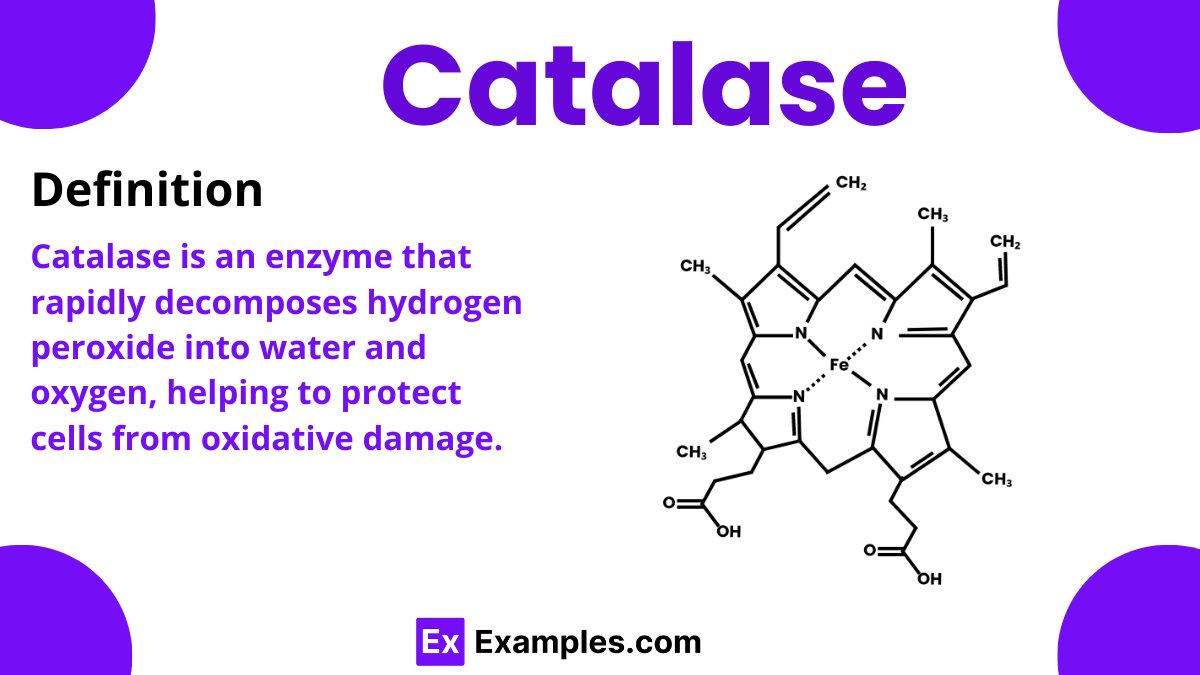
Catalase is a fascinating enzyme found in nearly all living organisms that are exposed to oxygen. This complex compound plays a critical role in protecting cells by breaking down hydrogen peroxide, a potentially harmful byproduct of cellular processes, into water and oxygen. By doing so, catalase helps maintain cellular health and prevents damage to tissues. This enzyme is essential in the study of chemistry, as it showcases how specific proteins work within living systems to manage chemical reactions. Its ability to rapidly convert harmful substances into harmless ones highlights the importance of biochemical processes in our daily lives.
Catalase enzymes vary slightly depending on the organism they are found in, but primarily, they can be classified into three main types: typical catalases, catalase-peroxidases, and manganese catalases. Each type serves the fundamental role of breaking down hydrogen peroxide into water and oxygen but differs slightly in their structure and the additional functions they perform.
Typical catalases are the most common form found in animals, plants, and fungi. They are highly efficient and focus solely on converting hydrogen peroxide into less harmful substances to prevent cell damage.
Catalase-peroxidases are more versatile and found mainly in bacteria and archaea. In addition to breaking down hydrogen peroxide, they can also act on other peroxides, serving a dual function which makes them crucial for the survival of these organisms in harsh environments.
Manganese catalases, instead of containing iron in their active sites like the other types, contain manganese. This type is less common and is typically found in bacteria that need to adapt to very high levels of hydrogen peroxide. Manganese catalases help these bacteria manage oxidative stress differently from organisms that contain the more typical iron-based catalases.
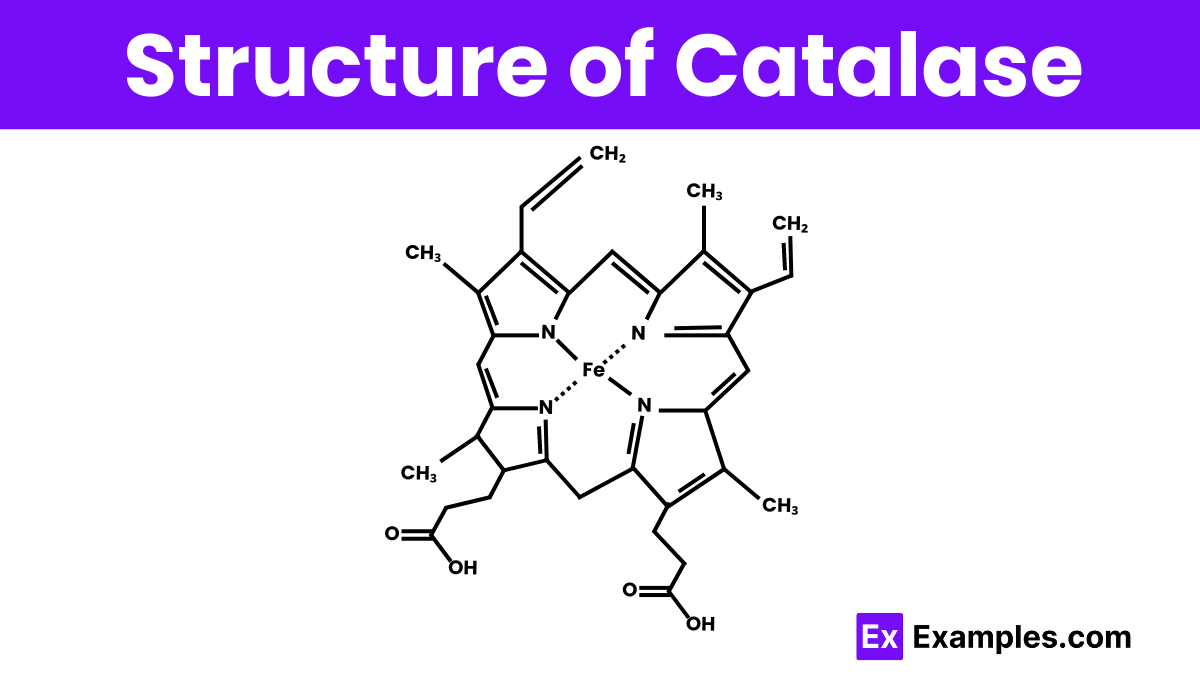
Catalase is a large, complex enzyme made up of four identical subunits, each containing an iron-containing heme group. This heme group is crucial for the enzyme’s ability to process hydrogen peroxide into water and oxygen. Each subunit is not just a simple chain but folds into a unique shape that allows the enzyme to function effectively. The structure of catalase is designed to maximize its efficiency, allowing it to react with millions of hydrogen peroxide molecules every second. This makes catalase extremely effective at protecting cells from oxidative damage.
| Property | Description |
|---|---|
| Molecular Weight | Approximately 250,000 grams per mole; varies slightly depending on the source organism. |
| Optimal pH | Ranges from 7.0 to 11.0, optimal around pH 7.0 for most catalases. |
| Optimal Temperature | Generally 37°C (98.6°F), though stability can range from 30°C to 60°C (86°F to 140°F). |
| Solubility | Soluble in water, facilitating its function in the cellular environment. |
| Stability | Stable under normal physiological conditions; loses activity when exposed to high concentrations of hydrogen peroxide. |
| Appearance | Colorless to slightly brown, crystalline solid when purified. |
Catalase significantly accelerates the decomposition of hydrogen peroxide into water and oxygen, a crucial reaction for shielding cellular components from oxidative harm. The reaction can be summarized by the chemical equation:
This illustrates how catalase converts two molecules of hydrogen peroxide into two molecules of water and one molecule of oxygen.
Although primarily known for its action on hydrogen peroxide, catalase can occasionally catalyze the oxidation of other small molecules in the presence of hydrogen peroxide, thereby acting as a peroxidase.
The active site in catalase contains a heme group, central to which is an iron ion. This iron is essential for the enzyme’s interaction with hydrogen peroxide and alternates between the Fe(III) and Fe(IV) oxidation states during the reaction process.
Catalase operates best within a specific pH range, generally around pH 7.0, showing optimal activity. Its effectiveness decreases when the pH deviates from this range, highlighting its sensitivity to environmental changes.
Catalase maintains stability and remains active within certain temperature limits, usually around 37°C (98.6°F), which aligns with the body temperature of many living organisms, including humans.
The primary function of catalase is to catalyze the rapid decomposition of hydrogen peroxide into water and oxygen. This is vital because hydrogen peroxide is a toxic byproduct of many metabolic reactions and can cause cellular damage if not removed.
By breaking down hydrogen peroxide, catalase helps protect cells from oxidative stress. This stress can lead to cell death and has been linked to various diseases, including cancer and neurodegenerative disorders. Catalase mitigates these risks by ensuring that hydrogen peroxide levels remain low.
Recent studies suggest that catalase can also play a role in cellular signaling by modulating the levels of hydrogen peroxide. Hydrogen peroxide acts as a signaling molecule in various biological processes, and by controlling its concentration, catalase influences processes like cell growth, cell death, and response to stress.
Catalase acts as an antioxidant by removing hydrogen peroxide, thus preventing it from being converted into more reactive oxygen species (ROS) by other enzymes like myeloperoxidase. This activity contributes to the overall antioxidant defense system of the body, which protects against a range of oxidative damages.
The liver has lots of catalase. This enzyme breaks down hydrogen peroxide that forms when harmful substances are detoxified, helping to protect liver cells.
Red blood cells contain catalase. It protects these cells and the circulatory system from the hydrogen peroxide generated in metabolism.
Bacteria such as Escherichia coli, have catalase. It defends them from oxidative stress and attacks by the host’s immune system.
Catalase is in the peroxisomes of plant cells. It handles hydrogen peroxide from photorespiration, a process that involves taking in oxygen and releasing carbon dioxide in light.
Fungi like yeasts use catalase to manage oxidative stress during their metabolism, especially when they break down sugars without oxygen.
Catalase increases reaction rates by breaking down hydrogen peroxide into water and oxygen rapidly, enhancing metabolic efficiency.
The optimal temperature for catalase activity is around 37°C (98.6°F), typical for human body temperature.
Catalase functions best at a pH around 7.0, typical for most intracellular environments.
In food, catalase helps prevent oxidation, extending shelf life and preserving color and flavor.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary function of catalase in the cell?
DNA replication
Protein synthesis
Breakdown of hydrogen peroxide
Glucose metabolism
Catalase is found in which cellular organelle?
Mitochondria
Ribosome
Peroxisome
Nucleus
Which element is essential for the catalytic activity of catalase?
Magnesium
Zinc
Iron
Copper
What type of enzyme is catalase?
Oxidoreductase
Hydrolase
Lyase
Transferase
Catalase exhibits maximum activity at which pH range?
2-3
4-5
6-7
8-9
What is the byproduct of the catalase reaction besides water?
Carbon dioxide
Nitrogen
Oxygen
Ammonia
Which of the following organisms does NOT typically produce catalase?
Bacteria
Plants
Animals
Viruses
What is the molecular weight of catalase?
25 kDa
60 kDa
100 kDa
240 kDa
Catalase can protect cells from oxidative damage caused by:
Free radicals
Carbohydrates
Lipids
Proteins
Which test can be used to detect the presence of catalase in a sample?
Biuret test
Catalase test
Benedict's test
Iodine test
Before you leave, take our quick quiz to enhance your learning!

