What is the primary function of cellulose in plants?
Energy storage
Structural support
Transport of nutrients
Photosynthesis

Cellulose is a natural substance found in all plant cell walls, making it the most abundant organic compound on Earth. It is a complex carbohydrate, or polysaccharide, consisting of thousands of glucose units linked together. This structure gives cellulose its strength and rigidity, which is why it is crucial for maintaining the structure of plants. In everyday life, cellulose is used in making paper, certain fabrics like cotton, and even in the production of some biofuels. Despite being made of glucose, humans cannot digest cellulose due to the absence of the necessary enzyme to break it down, although it is an important dietary fiber in our diet.
Alpha cellulose is the highest grade of cellulose, which makes it extremely durable and resistant to acids. This quality makes it ideal for producing paper intended for long-term storage, such as archival papers and special-grade textiles. It’s highly valued in industries where longevity and stability of the material are crucial.
Beta cellulose ranks below alpha in terms of purity. It is more accessible and reactive, which limits its use to less durable products. Common applications include the making of standard papers and some textiles. Its properties make it fit for everyday use where the exceptional durability of alpha cellulose isn’t required.
Gamma cellulose is the least pure form and is distinguished by its solubility in a wider range of solutions than alpha or beta cellulose. This type of cellulose finds its niche in specific industrial uses where solubility and reactivity can be advantages, such as in certain process chemicals and lower-grade materials. Gamma cellulose’s versatility makes it valuable for specialized applications across various industries.
Cotton, a natural fiber found in clothing, is made of cellulose. This material makes it strong and durable.
Wood consists mainly of cellulose, giving it the strength needed for building and crafting. Trees use cellulose to maintain their structure.
Paper comes from cellulose fibers from wood pulp or other plants. It’s commonly used for writing, printing, and packaging because it’s abundant and adaptable.
Grasses like bamboo and sugarcane have cellulose in their structure, making them rigid. These plants are also useful in construction and other industries.
Fruits and vegetables such as celery, lettuce, and apples have cellulose in their structure, adding to their crispness.
Rayon, a soft and versatile semi-synthetic fabric, is made from Cellulose extracted from wood pulp. It’s used in clothing and upholstery.
Corn husks, rich in Cellulose, protect corn kernels and are used in crafts like corn husk dolls. They can also be composted.
| Property | Value |
|---|---|
| Name | Cellulose |
| Alternate Names | Alpha-Cel, Alpha-Cellulose, (1, 4-beta-D-glucosyl)(N) |
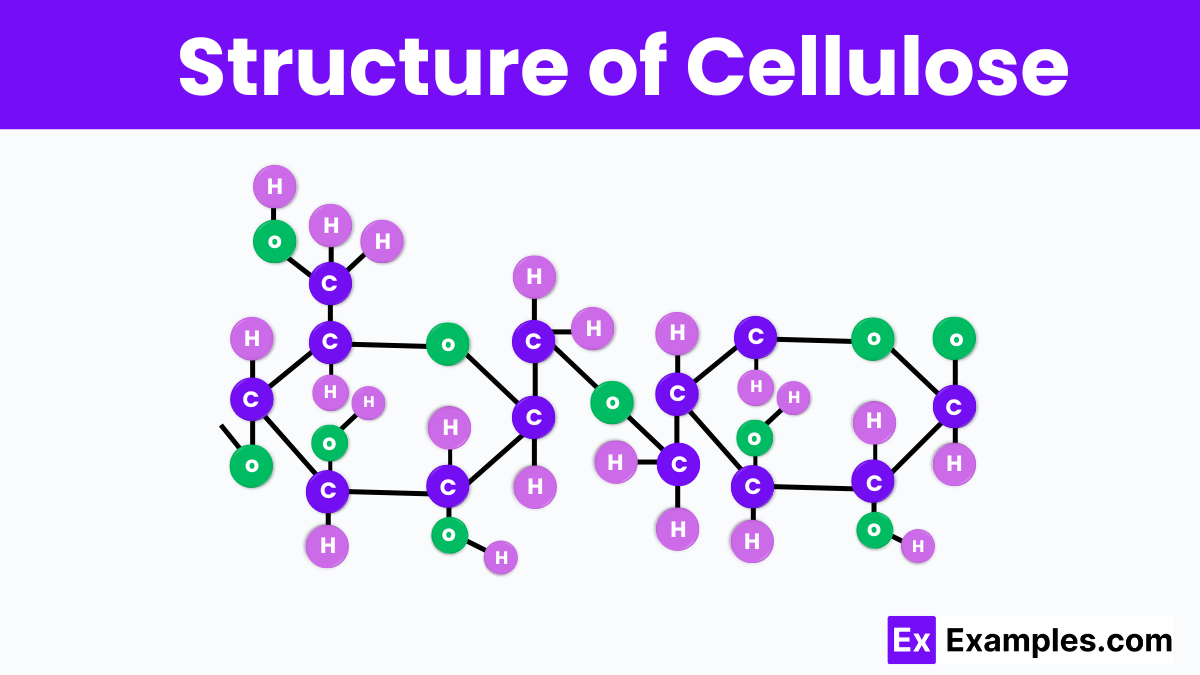
Cellulose has a unique and complex structure that is essential to plant strength and rigidity. It is composed of long chains of glucose molecules linked together by beta-1,4-glycosidic bonds. These chains bundle together to form microfibrils, which are tightly packed and hydrogen-bonded to each other, creating a strong, rigid framework that supports the plant cells. This linear and orderly arrangement allows cellulose to provide high tensile strength, which is why it’s a key material in nature, used in everything from the trees in our forests to the cotton in our clothes. Its structure not only supports plants but also has vast applications in industries due to its strength and natural abundance
Cellulose is not prepared artificial. in laboratories because it is a natural polymer produced by plants during the process of photosynthesis. The synthesis of Cellulose in plants involves the conversion of glucose into cellulose. This conversion can be summarized by the chemical equation:
In this process, multiple glucose molecules (C₆H₁₂O₆) undergo a dehydration reaction, where water molecules (H₂O) are removed, leading to the formation of long chains of Cellulose (C₆H₁₀O₅)ₙ. These chains are what form the structural components of the plant cell walls, providing rigidity and strength to the plant.
Although humans cannot integrate cellulose, they can extract it from plant material for various uses. The extraction involves treating plant fibers mechanically and chemically to separate cellulose from other plant components like lignin and hemicellulose. This purified cellulose is then used in numerous applications, from paper manufacturing to producing cellulose derivatives like cellulose acetate used in films and textiles.
| Property | Description |
|---|---|
| Appearance | White, fibrous, and odorless |
| Solubility | Insoluble in water and most organic solvents |
| Density | Approximately 1.5 g/cm³ |
| Melting Point | Does not melt; it decomposes at 260-270 °C |
| Tensile Strength | High; cellulose fibers can be extremely strong and durable |
| Thermal Stability | Stable up to 260 °C before decomposing |
Cellulose is a key ingredient in making paper, adding strength and flexibility to products like writing paper, cardboard, and specialty packaging.
Cellulose is turned into fibers used in the textile industry to make natural fabrics such as cotton, linen, and rayon. These materials are popular for clothing because they are comfortable, breathable, and eco-friendly.
Cellulose acts as a thickener, stabilizer, and emulsifier in foods. It also serves as a low-calorie filler in processed foods, helping to reduce calorie content without affecting taste or texture, as it is not digestible by humans.
Cellulose is important in the medical field for making various products, including wound dressings and filters. Oxidized cellulose is especially useful for its high absorbency, suitable for products like absorbent pads and surgical cotton.
Cellulose in food is good; it’s a dietary fiber that aids Digestion and supports gut health.
Cellulose acts as a fiber and Promoting Digestion health and Preventing Constipation.
Cellulose is neither a sugar nor a starch; it’s a complex carbohydrate known as a Polysaccharide.
Cellulose does not affect blood sugar levels as it is indigestible and does not convert to glucose.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary function of cellulose in plants?
Energy storage
Structural support
Transport of nutrients
Photosynthesis
What type of polymer is cellulose?
Polysaccharide
Protein
Lipid
Nucleic acid
Which of the following organisms can digest cellulose?
Humans
Cows
Fish
Birds
What type of bond connects the glucose units in cellulose?
α-1,4-glycosidic bond
α-1,6-glycosidic bond
β-1,4-glycosidic bond
β-1,6-glycosidic bond
Which enzyme is responsible for breaking down cellulose?
Amylase
Cellulase
Lactase
Lipase
What is the primary difference between cellulose and starch?
Solubility in water
Type of monomers
Presence of nitrogen
Type of glycosidic bond
Which industrial product is derived from cellulose?
Rayon
Nylon
Polyester
Acrylic
What is the main component of paper?
Starch
Cellulose
Hemicellulose
Lignin
Before you leave, take our quick quiz to enhance your learning!

