What is the atomic number of Cerium?
56
57
58
59
Dive into the comprehensive guide on Cerium, a cornerstone of the lanthanide series with far-reaching applications and fascinating chemistry. This guide unveils the essence of Cerium, from its basic definition to its multifaceted roles in enhancing modern technologies and its intriguing compounds. With practical examples, you’ll explore how Cerium contributes to environmental sustainability, medical advancements, and the technology sector, showcasing its indispensable presence in our daily lives and the future of innovation.
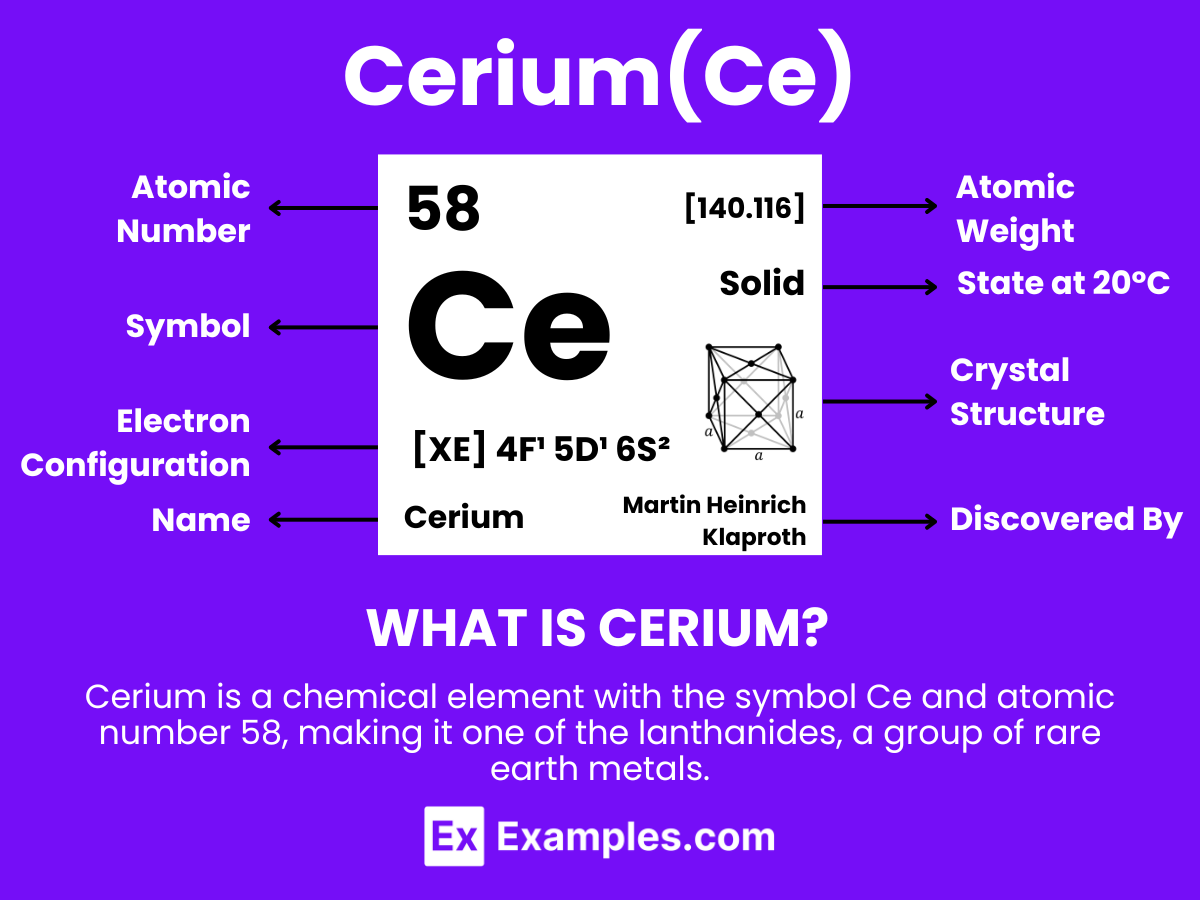
Cerium is a chemical element with the symbol Ce and atomic number 58, making it one of the lanthanides, a group of rare earth metals. It is a soft, silvery, ductile metal that tarnishes when exposed to air and is both malleable and relatively hard. Cerium has the distinction of being the most abundant of the rare earth elements, found in a variety of minerals including cerite, monazite, bastnäsite, and others.Cerium has notable chemical properties; it is especially reactive, easily oxidizing in the air and reacting with water. It exists in two oxidation states: +3 and +4, with the +4 state being more stable in oxygenated environments. This dual oxidative capacity enables cerium to act as a strong oxidizing agent in the +4 state, while in the +3 state, it behaves more like a typical rare earth metal.
Cerium (Ce) is an intriguing element in the lanthanide series of the periodic table, showcasing a unique atomic structure that underpins its diverse chemical behavior and applications. Here are the key aspects of Cerium’s atomic structure:
| Property | Value |
|---|---|
| Appearance | Silvery-white, malleable, ductile metal |
| Atomic Mass | 140.116 u |
| Density | 6.77 g/cm³ at 20 °C |
| Melting Point | 798 °C |
| Boiling Point | 3360 °C |
| Atomic Number | 58 |
| Atomic Radius | 185 pm |
| State at 20 °C | Solid |
| Crystal Structure | Face-centered cubic (fcc) |
| Electrical Resistivity | 828 nΩ·m at 20 °C |
| Thermal Conductivity | 11.3 W/(m·K) at 25 °C |
| Thermal Expansion | 6.3 µm/(m·K) at 25 °C |
Cerium, a rare earth metal, exhibits fascinating chemical properties due to its position in the lanthanide series. Its most common oxidation states are +3 (Ce³⁺) and +4 (Ce⁴⁺), with the +4 state being more stable in aqueous solutions. Cerium’s chemistry is characterized by its ability to readily switch between these oxidation states, which is utilized in various chemical reactions and applications.
Oxidation and Reduction:
Cerium can easily oxidize from the +3 to the +4 oxidation state, especially in aqueous solutions. This property makes it a strong oxidizing agent.
Ce³⁺ (aq) → Ce⁴⁺⁻
Conversely, Ce^4+ can act as an oxidizer, getting reduced to Ce^3+ in redox reactions.
Ce⁴⁺⁻ → Ce³+(aq)
Reaction with Water:
Cerium reacts with water, forming cerium(III) hydroxide and releasing hydrogen gas, especially when finely divided.
2Ce + 6H2O → 2Ce(OH)₃ + 3H2↑
Reaction with Acids:
Cerium metal dissolves in dilute acids, forming Ce³⁺ solutions.
Ce + 2HCl → CeCl₂ + H2↑
In more oxidizing acids like nitric acid, cerium can form Ce⁴⁺.
2Ce + 6HNO₃ → 2Ce(NO₃)₄ + 3H2O + 3NO2↑
Reaction with Oxygen:
At room temperature, cerium slowly tarnishes, forming a thin layer of cerium oxide (CeO₂), which prevents further oxidation.
4Ce + O2 → 2Ce2O₃
Upon heating, cerium burns vigorously to form CeO₂.
2Ce + O2 → 2CeO₂
Formation of Cerium Compounds:
Cerium forms a variety of compounds, both in +3 and +4 oxidation states, including oxides (Ce₂O₃ and CeO₂), sulfides (Ce₂S₃), and halides (CeF₃, CeCl₃, CeBr₃, CeI₃, etc.).
CeO₂, in particular, is widely used as a catalyst in automotive exhaust systems to reduce emissions and in chemical synthesis.
| Property | Value |
|---|---|
| Standard Atomic Weight | 140.116 u |
| Enthalpy of Fusion | 5.46 kJ/mol (at melting point) |
| Enthalpy of Vaporization | 398 kJ/mol (at boiling point) |
| Enthalpy of Atomization | 418 kJ/mol (at 25 °C) |
| Specific Heat Capacity | 26.94 J/(mol·K) (at 25 °C) |
| Thermal Conductivity | 11.3 W/(m·K) (at 25 °C) |
| Thermal Expansion | 6.3 µm/(m·K) (at 25 °C) |
| Property | Value |
|---|---|
| Young’s Modulus | 33.6 GPa |
| Shear Modulus | 13.5 GPa |
| Bulk Modulus | 21.5 GPa |
| Poisson’s Ratio | 0.24 |
| Mohs Hardness | 2.5 |
| Vickers Hardness | 270 MPa |
| Brinell Hardness | 412 MPa |
| Density | 6.77 g/cm³ (at 20 °C) |
| Property | Value |
|---|---|
| Electrical Resistivity | 828 nΩ·m (at 20 °C) |
| Magnetic Ordering | Paramagnetic (above 12 K) |
| Magnetic Susceptibility | +2730.0·10⁻⁶ cm³/mol (at 298 K) |
| Electrical Conductivity | About 1.25·10⁶ S/m |
| Property | Value |
|---|---|
| Natural Isotopes | ¹³⁶Ce, ¹³⁸Ce, ¹⁴⁰Ce, ¹⁴²Ce |
| Most Stable Isotopes | ¹⁴⁰Ce (stable), ¹⁴²Ce (stable), ¹³⁸Ce (stable), ¹³⁶Ce (stable) |
| Isotopic Abundance | ¹⁴⁰Ce (88.48%), ¹⁴²Ce (11.08%), ¹³⁸Ce (0.25%), ¹³⁶Ce (0.19%) |
| Neutron Cross Section | ¹⁴⁰Ce: 0.57 barns (thermal neutrons) |
| Neutron Mass Absorption | 0.00013 |
| Atomic Number | 58 |
| Half-life of Most Unstable Isotope | ¹⁵²Ce (half-life: ~8.4 years) |
Cerium is predominantly obtained from cerium-rich minerals like monazite and bastnasite, which contain a mixture of rare earth elements. The extraction and preparation process involves several steps:
1.Cerium(III) Oxide (Ce₂O₃):
2.Cerium(IV) Oxide (CeO₂):
3.Cerium(III) Chloride (CeCl₃):
4.Cerium(IV) Sulfate (Ce(SO₄)₂):
5.Cerium(III) Nitrate (Ce(NO₃)₃):
6.Cerium(IV) Bromide (CeBr₄CeBr₄):
| Isotope | Natural Abundance (%) | Half-life | Decay Mode |
|---|---|---|---|
| ¹³⁶Ce | 0.185 | Stable | – |
| ¹³⁸Ce | 0.251 | Stable | – |
| ¹⁴⁰Ce | 88.450 | Stable | – |
| ¹⁴²Ce | 11.114 | Stable | – |
| ¹⁴⁶Ce | – | 284.893 days | Beta decay to ¹⁴⁴Pr |
Cerium, a versatile and abundant rare earth element, has a wide range of applications across various industries due to its unique physical and chemical properties. Some of the primary uses of cerium include:
The production of cerium involves several key processes, beginning with the extraction from rare earth mineral ores, such as monazite and bastnasite, which contain a mix of cerium and other lanthanides. The basic steps in cerium production include:
Cerium and its compounds have a wide range of applications, reflecting the metal’s versatile properties:
Cerium, a versatile and abundant element in the Earth’s crust, plays a crucial role in modern technology and industry. Its unique physical, chemical, and nuclear properties enable its use in catalysis, glass polishing, and as an alloying agent, among others. Understanding cerium’s isotopes, including both stable and radioactive varieties, provides insight into its applications and environmental impact.

Dive into the comprehensive guide on Cerium, a cornerstone of the lanthanide series with far-reaching applications and fascinating chemistry. This guide unveils the essence of Cerium, from its basic definition to its multifaceted roles in enhancing modern technologies and its intriguing compounds. With practical examples, you’ll explore how Cerium contributes to environmental sustainability, medical advancements, and the technology sector, showcasing its indispensable presence in our daily lives and the future of innovation.
Cerium is a chemical element with the symbol Ce and atomic number 58, making it one of the lanthanides, a group of rare earth metals. It is a soft, silvery, ductile metal that tarnishes when exposed to air and is both malleable and relatively hard. Cerium has the distinction of being the most abundant of the rare earth elements, found in a variety of minerals including cerite, monazite, bastnäsite, and others.Cerium has notable chemical properties; it is especially reactive, easily oxidizing in the air and reacting with water. It exists in two oxidation states: +3 and +4, with the +4 state being more stable in oxygenated environments. This dual oxidative capacity enables cerium to act as a strong oxidizing agent in the +4 state, while in the +3 state, it behaves more like a typical rare earth metal.
Formula: Ce
Composition: Composed of a single cerium atom.
Bond Type: In its elemental form, cerium does not engage in bonding as it exists as a pure element. However, cerium is capable of forming both covalent and ionic bonds when interacting with other elements, enabling the formation of a variety of compounds.
Molecular Structure: Cerium, as an individual element, does not form traditional molecular structures in its pure form. It is expected to exhibit the characteristics of a typical soft metal, likely with a face-centered cubic crystalline structure in its solid state.
Electron Sharing: Cerium participates in electron sharing through covalent bonds or electron transfer in ionic bonds with different elements. It primarily forms trivalent Ce³⁺ ions in its compounds, though it can also assume a +4 oxidation state in cerium(IV) compounds.
Significance: Cerium is essential in numerous fields, including as a catalyst in automotive catalytic converters, in the polishing of glass and mirrors, and in the manufacturing of phosphors for electronics. Its oxides are particularly valued in various industrial and technological applications.
Role in Chemistry: Beyond its practical uses, cerium plays a crucial role in the study of lanthanide series chemistry, offering insights into the behavior of rare earth metals and their chemical interactions. Its ability to exist in multiple oxidation states underscores the complexity and significance of cerium in modern science and technology.
Cerium (Ce) is an intriguing element in the lanthanide series of the periodic table, showcasing a unique atomic structure that underpins its diverse chemical behavior and applications. Here are the key aspects of Cerium’s atomic structure:
Atomic Number: 58, indicating it has 58 protons in its nucleus and, in a neutral atom, 58 electrons orbiting around the nucleus.
Atomic Mass: Approximately 140.116 atomic mass units (amu), reflecting the average mass of its isotopes and their relative abundance.
Electron Configuration: [Xe] 4f¹ 5d¹ 6s². This configuration reveals that Cerium’s electrons fill up to the 6s orbital, with one electron in the 4f orbital and another in the 5d orbital, illustrating its position as a transition metal within the f-block of the periodic table.
Oxidation States: Cerium predominantly exhibits two oxidation states in its compounds: +3 (Ce³⁺) and +4 (Ce⁴⁺), with the +4 state being more stable in oxygenated environments, a unique characteristic among the lanthanides.
Electronegativity: On the Pauling scale, Cerium has an electronegativity of approximately 1.12, indicating a relatively low tendency to attract electrons within a chemical bond.
Atomic Radius: The atomic radius of Cerium is about 182.5 picometers (pm), which is typical for lanthanides and reflects its size relative to other elements in the periodic table.
Ionization Energies: The first ionization energy of Cerium is about 534.4 kJ/mol, which is the energy required to remove the most loosely bound electron from an atom of Cerium.
Property | Value |
|---|---|
Appearance | Silvery-white, malleable, ductile metal |
Atomic Mass | 140.116 u |
Density | 6.77 g/cm³ at 20 °C |
Melting Point | 798 °C |
Boiling Point | 3360 °C |
Atomic Number | 58 |
Atomic Radius | 185 pm |
State at 20 °C | Solid |
Crystal Structure | Face-centered cubic (fcc) |
Electrical Resistivity | 828 nΩ·m at 20 °C |
Thermal Conductivity | 11.3 W/(m·K) at 25 °C |
Thermal Expansion | 6.3 µm/(m·K) at 25 °C |
Cerium, a rare earth metal, exhibits fascinating chemical properties due to its position in the lanthanide series. Its most common oxidation states are +3 (Ce³⁺) and +4 (Ce⁴⁺), with the +4 state being more stable in aqueous solutions. Cerium’s chemistry is characterized by its ability to readily switch between these oxidation states, which is utilized in various chemical reactions and applications.
Oxidation and Reduction:
Cerium can easily oxidize from the +3 to the +4 oxidation state, especially in aqueous solutions. This property makes it a strong oxidizing agent.
Ce³⁺ (aq) → Ce⁴⁺⁻
Conversely, Ce^4+ can act as an oxidizer, getting reduced to Ce^3+ in redox reactions.
Ce⁴⁺⁻ → Ce³+(aq)
Reaction with Water:
Cerium reacts with water, forming cerium(III) hydroxide and releasing hydrogen gas, especially when finely divided.
2Ce + 6H2O → 2Ce(OH)₃ + 3H2↑
Reaction with Acids:
Cerium metal dissolves in dilute acids, forming Ce³⁺ solutions.
Ce + 2HCl → CeCl₂ + H2↑
In more oxidizing acids like nitric acid, cerium can form Ce⁴⁺.
2Ce + 6HNO₃ → 2Ce(NO₃)₄ + 3H2O + 3NO2↑
Reaction with Oxygen:
At room temperature, cerium slowly tarnishes, forming a thin layer of cerium oxide (CeO₂), which prevents further oxidation.
4Ce + O2 → 2Ce2O₃
Upon heating, cerium burns vigorously to form CeO₂.
2Ce + O2 → 2CeO₂
Formation of Cerium Compounds:
Cerium forms a variety of compounds, both in +3 and +4 oxidation states, including oxides (Ce₂O₃ and CeO₂), sulfides (Ce₂S₃), and halides (CeF₃, CeCl₃, CeBr₃, CeI₃, etc.).
CeO₂, in particular, is widely used as a catalyst in automotive exhaust systems to reduce emissions and in chemical synthesis.
Property | Value |
|---|---|
Standard Atomic Weight | 140.116 u |
Enthalpy of Fusion | 5.46 kJ/mol (at melting point) |
Enthalpy of Vaporization | 398 kJ/mol (at boiling point) |
Enthalpy of Atomization | 418 kJ/mol (at 25 °C) |
Specific Heat Capacity | 26.94 J/(mol·K) (at 25 °C) |
Thermal Conductivity | 11.3 W/(m·K) (at 25 °C) |
Thermal Expansion | 6.3 µm/(m·K) (at 25 °C) |
Property | Value |
|---|---|
Young’s Modulus | 33.6 GPa |
Shear Modulus | 13.5 GPa |
Bulk Modulus | 21.5 GPa |
Poisson’s Ratio | 0.24 |
Mohs Hardness | 2.5 |
Vickers Hardness | 270 MPa |
Brinell Hardness | 412 MPa |
Density | 6.77 g/cm³ (at 20 °C) |
Property | Value |
|---|---|
Electrical Resistivity | 828 nΩ·m (at 20 °C) |
Magnetic Ordering | Paramagnetic (above 12 K) |
Magnetic Susceptibility | +2730.0·10⁻⁶ cm³/mol (at 298 K) |
Electrical Conductivity | About 1.25·10⁶ S/m |
Property | Value |
|---|---|
Natural Isotopes | ¹³⁶Ce, ¹³⁸Ce, ¹⁴⁰Ce, ¹⁴²Ce |
Most Stable Isotopes | ¹⁴⁰Ce (stable), ¹⁴²Ce (stable), ¹³⁸Ce (stable), ¹³⁶Ce (stable) |
Isotopic Abundance | ¹⁴⁰Ce (88.48%), ¹⁴²Ce (11.08%), ¹³⁸Ce (0.25%), ¹³⁶Ce (0.19%) |
Neutron Cross Section | ¹⁴⁰Ce: 0.57 barns (thermal neutrons) |
Neutron Mass Absorption | 0.00013 |
Atomic Number | 58 |
Half-life of Most Unstable Isotope | ¹⁵²Ce (half-life: ~8.4 years) |
Cerium is predominantly obtained from cerium-rich minerals like monazite and bastnasite, which contain a mixture of rare earth elements. The extraction and preparation process involves several steps:
Mining and Initial Processing: The ore (monazite or bastnasite) is mined and subjected to a preliminary treatment to obtain a concentrate of rare earth minerals.
Digestion: The concentrate is treated with a suitable acid (hydrochloric acid or sulfuric acid) to produce a solution of rare earth chlorides or sulfates.
Solvent Extraction: The solution undergoes solvent extraction, a process that separates cerium and other rare earth elements based on their chemical properties and solubility in different solvents.
Precipitation and Calcination: Cerium is selectively precipitated from the mixture by adjusting the pH. The precipitate is then calcined (heated) to obtain cerium oxide (CeO2).
Metallic Cerium Production: To produce metallic cerium, the cerium oxide is reduced using a suitable reducing agent like lanthanum or mischmetal. Electrolysis can also be used for reduction, involving the conversion of cerium oxide to cerium fluoride and then electrolyzing it in a molten salt solution.
1.Cerium(III) Oxide (Ce₂O₃):
Obtained by the reduction of CeO₂ in a hydrogen atmosphere.
CeO₂+H₂→Ce₂O₃+H₂O
2.Cerium(IV) Oxide (CeO₂):
Used as a catalyst and in polishing materials. Prepared by heating cerium(III) nitrate.
2Ce(NO₃)₃ →2CeO₂+6NO₂+O₂
3.Cerium(III) Chloride (CeCl₃):
Prepared by the reaction of cerium metal with chlorine gas.
2Ce+3Cl₂→2CeCl₃
4.Cerium(IV) Sulfate (Ce(SO₄)₂):
A powerful oxidizing agent, especially in sulfuric acid solution.
Ce+2H₂SO₄→Ce(SO₄)₂ + 2H₂
5.Cerium(III) Nitrate (Ce(NO₃)₃):
Used in the production of CeO₂ and other cerium compounds.
Ce+4HNO₃→Ce(NO₃)₃+2H₂O+NO₂
6.Cerium(IV) Bromide (CeBr₄CeBr₄):
Formed by the reaction of cerium(III) bromide with bromine gas.
2CeBr₃+Br₂→2CeBr₄
Isotope | Natural Abundance (%) | Half-life | Decay Mode |
|---|---|---|---|
¹³⁶Ce | 0.185 | Stable | – |
¹³⁸Ce | 0.251 | Stable | – |
¹⁴⁰Ce | 88.450 | Stable | – |
¹⁴²Ce | 11.114 | Stable | – |
¹⁴⁶Ce | – | 284.893 days | Beta decay to ¹⁴⁴Pr |
Cerium, a versatile and abundant rare earth element, has a wide range of applications across various industries due to its unique physical and chemical properties. Some of the primary uses of cerium include:
Catalysis: Cerium oxide (CeO2) is widely used as a catalyst in automotive exhaust systems to reduce harmful emissions such as carbon monoxide, nitrogen oxides, and hydrocarbons, converting them into less harmful gases (CO2, N2, and H2O).
Glass Polishing: Cerium oxide is a key ingredient in glass-polishing powders and compounds due to its effectiveness in removing minor scratches and marks from glass surfaces, including mirrors, lenses, and television screens.
Glass Additive: Adding cerium to glass can improve its color and UV resistance. This is particularly useful in eyeglass lenses to protect eyes from UV rays and in the glass covers of solar panels to prevent UV degradation.
Phosphors: Cerium is used in the production of phosphors that are essential for the manufacturing of fluorescent lamps and LED lights. Cerium-doped materials emit light when exposed to electrons or ultraviolet light, making them key components in display and lighting technologies.
Steel Manufacturing: In the steel industry, cerium is used as a deoxidizer and decarbonizer to improve the quality of the steel. It helps in removing impurities and refining the steel’s grain structure.
Alloying Agent: Cerium, when alloyed with other metals such as aluminum and iron, can enhance the properties of the alloys, making them more resistant to oxidation and improving their strength at high temperatures.
Environmental Applications: Due to its oxidative properties, cerium is explored for use in water splitting processes to produce hydrogen, a potential clean energy source. It is also used in photocatalysts for breaking down organic pollutants in water and air.
Chemical Manufacturing: Cerium compounds are used in various chemical reactions and manufacturing processes, including as a catalyst in organic synthesis and in the production of pigments and ceramics.
The production of cerium involves several key processes, beginning with the extraction from rare earth mineral ores, such as monazite and bastnasite, which contain a mix of cerium and other lanthanides. The basic steps in cerium production include:
Ore Processing: The ore is crushed and ground, then subjected to physical or chemical treatments to increase the concentration of rare earth elements.
Extraction: The concentrated ore is treated with acid to convert the rare earth elements into soluble salts.
Separation: The rare earth elements are separated from each other using solvent extraction or ion exchange techniques. This step is complex due to the similar chemical properties of lanthanides.
Refinement: The separated elements, including cerium, are further refined to remove impurities, typically resulting in cerium oxide (CeO2) or cerium carbonate (Ce2(CO3)3).
Metal Production: If metallic cerium is required, cerium oxide is reduced using a suitable reducing agent such as calcium metal in a process known as metallothermic reduction.
Cerium and its compounds have a wide range of applications, reflecting the metal’s versatile properties:
Catalysis: Cerium oxide (CeO2) is used as a catalyst in automotive exhaust systems to convert harmful emissions into less toxic gases. It’s also used in the petroleum industry for refining crude oil.
Glass and Ceramics: Cerium is used in glass polishing and manufacturing, where cerium oxide adds color and UV resistance. It’s also used in the production of fluorescent glasses and ceramics.
Electronics and Semiconductors: Cerium compounds are used in the manufacturing of phosphors for color television screens and fluorescent lamps.
Steel Manufacturing: Cerium is used as a deoxidizer and decarbonizer in steel production, improving the quality of the steel.
Environmental Protection: Cerium compounds are utilized in water splitting to produce hydrogen, a clean energy source, and in photocatalysis for degrading organic pollutants in water.
Others: Cerium’s unique properties make it useful in self-cleaning ovens, carbon arc lighting, and in various chemical reactions as a reagent or catalyst.
Cerium, a versatile and abundant element in the Earth’s crust, plays a crucial role in modern technology and industry. Its unique physical, chemical, and nuclear properties enable its use in catalysis, glass polishing, and as an alloying agent, among others. Understanding cerium’s isotopes, including both stable and radioactive varieties, provides insight into its applications and environmental impact.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Cerium?
56
57
58
59
What is the chemical symbol for Cerium?
Ce
Cr
Cs
Cm
Cerium belongs to which group of elements?
Transition metals
Alkali metals
Lanthanides
Actinides
What is the most common oxidation state of Cerium in its compounds?
+1
+2
+3
+4
Which mineral is the primary source of Cerium?
Bauxite
Galena
Hematite
Monazite
Which of the following is a common use of Cerium oxide?
Fertilizers
Water purification
Abrasives in polishing
Building materials
Cerium is used in which component of automotive catalytic converters?
Catalyst support
Exhaust pipes
Muffler
Fuel tank
What is the melting point of Cerium?
798°C
795°C
840°C
650°C
Cerium is used in flints for which purpose?
Enhancing durability
Increasing flexibility
Preventing rust
Producing sparks
Which of the following is NOT a property of Cerium?
Soft and malleable
Silvery-white
Highly reactive
Brittle
Before you leave, take our quick quiz to enhance your learning!

