What is the atomic number of Chromium?
22
24
26
28
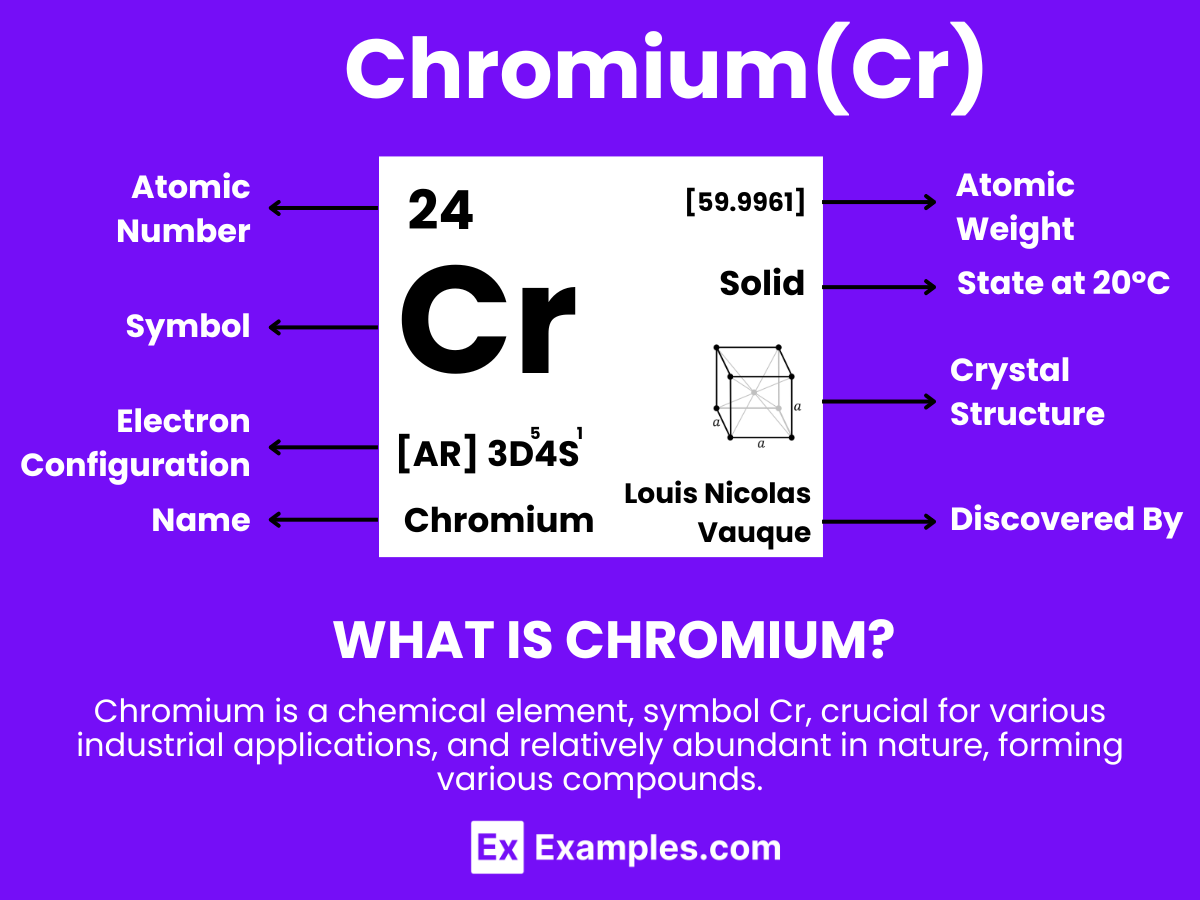
Chromium, a lustrous, steely-gray metal, plays a pivotal role in both health and technological advancements. This guide delves into the multifaceted uses and benefits of chromium, from its essential contribution to metabolic processes in the human body to its critical application in manufacturing and industrial sectors. With a focus on how chromium enhances the durability of materials and supports nutritional well-being, we explore its significance in daily life and innovative technologies. Through detailed examples, this article sheds light on chromium’s indispensable presence in our modern world.
Chromium is a lustrous, steel-gray metal that stands out for its durability and shiny appearance. Holding the atomic number 24. chromium is renowned for its high corrosion resistance and hardness. This metal is not found freely in nature but is primarily extracted from the mineral chromite. Chromium is extensively utilized across numerous industries, owing to its versatile properties. It plays a crucial role in the manufacturing sector, especially in metal plating and as an important component in stainless steel, enhancing its corrosion resistance. Additionally, chromium is used in the production of dyes and pigments, given its vibrant and various colors when combined with other elements. Its application extends to the leather tanning industry as well, where it is used to produce durable and resistant leather products
| Property | Value |
|---|---|
| Appearance | Lustrous, steel-gray metal |
| Atomic Number | 24 |
| Atomic Weight | 51.9961 |
| Density | 7.15 g/cm³ at 20°C |
| Melting Point | 1907°C (3465°F) |
| Boiling Point | 2671°C (4840°F) |
| State at 20 °C | Solid |
| Electrical Conductivity | Good, but less than copper |
| Thermal Conductivity | 93.9 W/(m·K) at 25°C |
| Heat of Fusion | 21.0 kJ/mol |
| Heat of Vaporization | 347 kJ/mol |
| Specific Heat Capacity | 0.449 J/g·K at 25°C |
| Electronegativity | 1.66 (Pauling scale) |
| Crystal Structure | Body-centered cubic (bcc) |
Chromium, a transition metal with the symbol Cr and atomic number 24, exhibits a wide array of chemical properties owing to its position in the periodic table. This element is known for its remarkable resistance to tarnishing and high melting point, characteristics that make it invaluable across various industrial applications.
Chromium displays a variety of oxidation states, ranging from -2 to +6, with +3 (Cr^3+) and +6 (Cr^6+) being the most stable and common. These varied oxidation states allow chromium to form a diverse range of compounds, reflecting its versatile chemistry.
One of chromium’s most notable chemical properties is its exceptional resistance to corrosion. This is largely due to the formation of a thin, protective oxide layer on the surface when exposed to oxygen. This layer, primarily consisting of chromium(III) oxide (Cr₂O₃), acts as a barrier that prevents further oxidation of the underlying metal.
Chromium reacts with acids to form chromium salts and hydrogen gas, showcasing its reactivity with different chemical reagents. For example, when chromium reacts with hydrochloric acid, the reaction yields chromium(III) chloride and hydrogen:
2 Cr+6 HCl→2 CrCl₃+3 H₂
In the presence of bases and oxidizing agents, chromium can form chromate and dichromate ions, which are in the +6 oxidation state. For instance, when chromium(III) oxide reacts with sodium carbonate in the presence of oxygen, sodium chromate is formed:
2 Cr₂O₃+4 Na₂CO₃+3 O₂→4 Na₂CrO₄+4 CO₂
Chromium compounds, especially those in the hexavalent state are toxic and have significant environmental and health impacts. Chromium(VI) compounds, such as chromium trioxide and various chromates, are powerful oxidizing agents and are carcinogenic. Proper handling and disposal practices are essential to mitigate their adverse effects.
| Property | Value |
|---|---|
| Melting Point | 1907 °C |
| Boiling Point | 2671 °C |
| Heat of Fusion | 21.0 kJ/mol |
| Heat of Vaporization | 347 kJ/mol |
| Specific Heat Capacity | 0.449 J/g·K |
| Thermal Conductivity | 93.9 W/m·K |
| Thermal Expansion | 4.9 µm/m·K (at 25 °C) |
| Property | Value |
|---|---|
| Density | 7.19 g/cm^3 |
| Mohs Hardness | 8.5 |
| Young’s Modulus | 279 GPa |
| Tensile Strength | 282 MPa |
| Brinell Hardness | 687 HB |
| Poisson’s Ratio | 0.21 |
| Property | Value |
|---|---|
| Electrical Resistivity | 125 nΩ·m (at 20 °C) |
| Magnetic Ordering | Antiferromagnetic (below 311 K) |
| Magnetic Susceptibility | -98.6·10^-6 cm^3/mol (at 298 K) |
| Property | Value |
|---|---|
| Natural Isotopes | ^50Cr, ^52Cr, ^53Cr, ^54Cr |
| Isotope Abundance | ^50Cr (4.345%), ^52Cr (83.789%), ^53Cr (9.501%), ^54Cr (2.365%) |
| Atomic Mass | 51.9961 u |
| Cross Section | 18.1 barns (for ^52Cr, thermal neutrons) |
| Half-Life | Stable (most isotopes) |
The preparation of chromium involves several key steps to extract it from its ores and refine it into a usable form. The most common ore of chromium is chromite (FeCr₂O₄ ), from which chromium is extracted through various processes. Here is an overview of the primary methods used in the preparation of chromium:
Chromium has several isotopes, both stable and radioactive. Below is a table summarizing the most notable isotopes of chromium, including their mass numbers and key characteristics.
| Isotope | Mass Number | Natural Abundance (%) | Half-Life | Notes |
|---|---|---|---|---|
| Cr-50 | 50 | 4.345 | Stable | Used in isotopic geological dating |
| Cr-52 | 52 | 83.789 | Stable | Most abundant chromium isotope |
| Cr-53 | 53 | 9.501 | Stable | Used in environmental and geological studies |
| Cr-54 | 54 | 2.365 | Stable | – |
| Cr-51 | 51 | – | 27.7 days | Used in medical and industrial applications as a tracer |
Stable isotopes of chromium play significant roles in various scientific studies due to their unique properties. Meanwhile, the radioactive chromium-51 has practical applications in industry and medicine, particularly due to its relatively short half-life and the ability to be used as a tracer.
Chromium is a versatile metal with numerous applications that take advantage of its unique properties, such as corrosion resistance, hardness, and shiny appearance. Below are some of the primary uses of chromium:
Chromium production begins with the mining of chromite ore (FeCr2O4), which is the main source of chromium. This process involves extracting chromite from open pit or underground mines. After mining, the chromite ore is initially concentrated through various processes including crushing, grinding, and gravity separation to enhance its chromium content.
The concentrated chromite ore is then subjected to a smelting process in a furnace, where it is mixed with carbon and lime. The high temperatures in the furnace lead to the reduction of the chromite ore, resulting in the production of ferrochrome, an alloy of iron and chromium. This ferrochrome is the primary form of chromium used in various industries. The exact conditions within the furnace, including temperature and time, are carefully controlled to produce ferrochrome with different chromium contents, depending on the requirements of the final applications.
Environmental considerations are also a significant part of the chromium production process. The mining and smelting processes can lead to the release of harmful pollutants, including chromium VI, a known carcinogen. As such, modern production facilities implement various measures to reduce emissions and manage waste, including water and air filtration systems.
Chromium is a versatile element with various applications across many industries due to its unique properties, such as high corrosion resistance, hardness, and the ability to form a shiny, reflective surface when polished.
Chromium’s distinctive properties, such as its corrosion resistance, high melting point, and brilliant shine, make it indispensable in stainless steel production and decorative applications. This overview underscores the metal’s versatility and essential contribution to advancements in materials science and engineering, highlighting its significance in both historical and contemporary contexts.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Chromium?
22
24
26
28
What is the chemical symbol for Chromium?
Cr
Cm
Co
Cf
Chromium is primarily used in which of the following applications?
Fertilizers
Stainless steel production
Pharmaceuticals
Food additives
What is the most common oxidation state of Chromium in its compounds?
+1
+2
+3
+4
Which of the following is a characteristic property of Chromium?
High melting point
Low density
Poor electrical conductivity
Brittle
Which process is commonly used to extract Chromium from its ore?
Bayer process
Electrolysis
Roasting and leaching
Smelting
What is the role of Chromium in the human body?
DNA synthesis
Blood clotting
Glucose metabolism
Nerve function
Which of the following is a Chromium compound used in tanning leather?
Chromium(III) chloride
Chromium(III) oxide
Chromium(III) sulfate
Chromium(III) nitrate
Chromium can exhibit various oxidation states. Which of the following is NOT a common oxidation state of Chromium?
+2
+4
+6
+8
Which color is associated with Chromium(III) compounds?
Blue
Green
Yellow
Red
Before you leave, take our quick quiz to enhance your learning!

