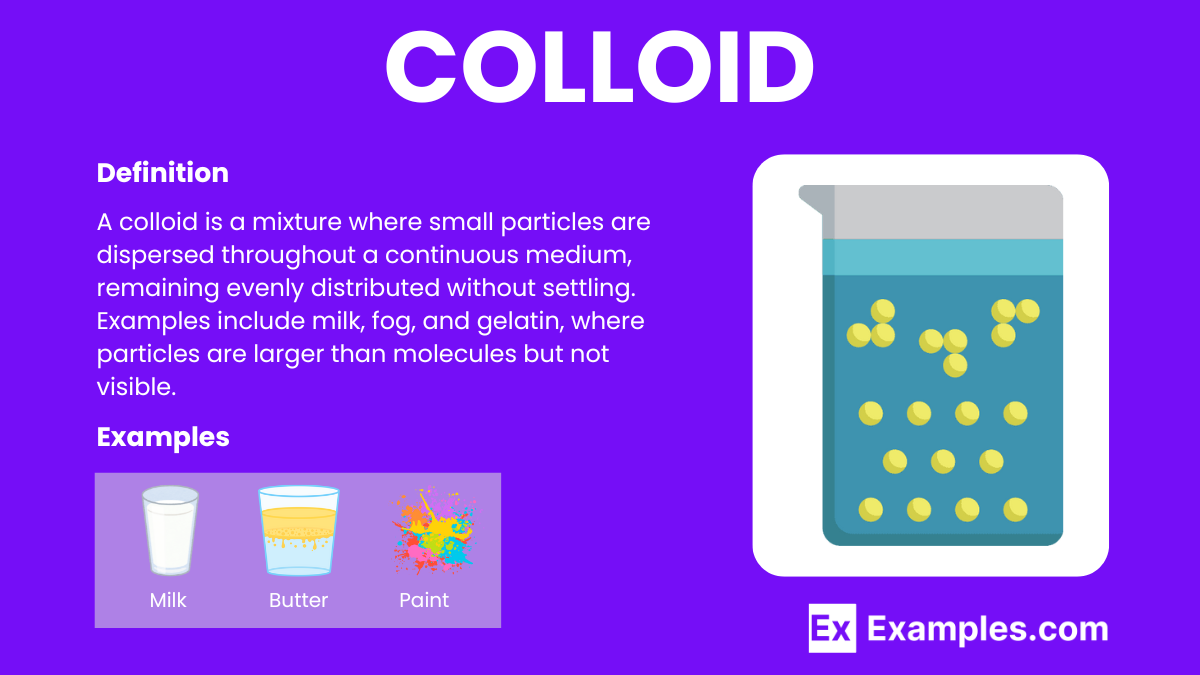
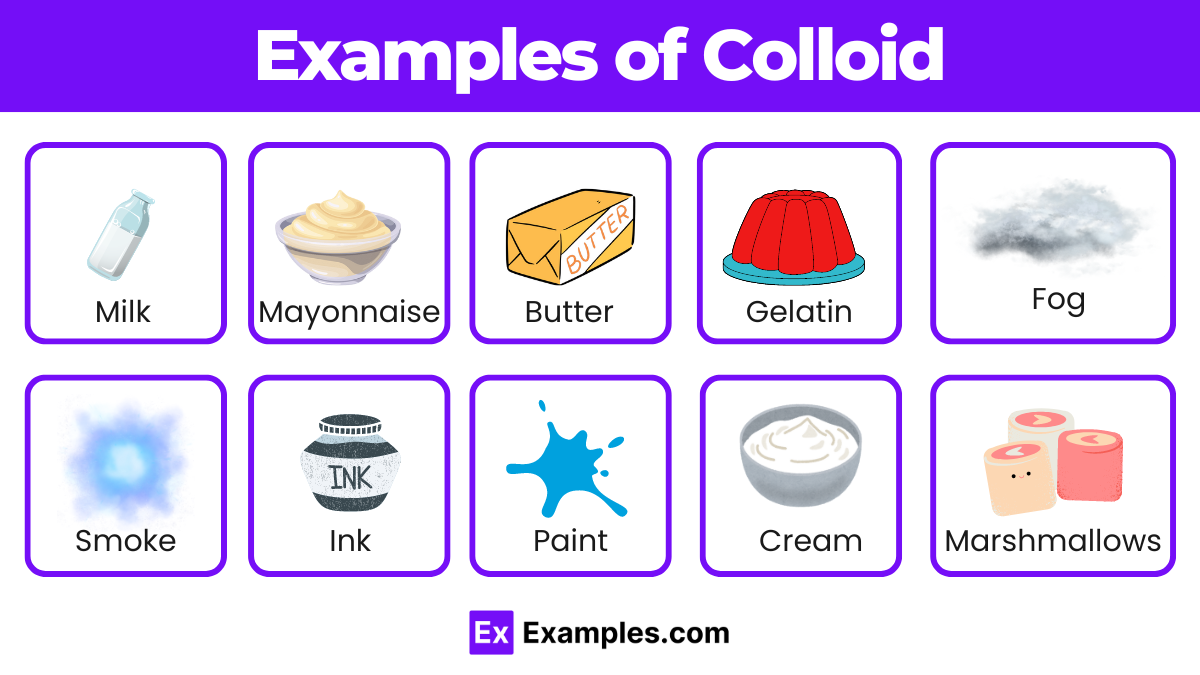
What is a colloid?
A solution where particles are dissolved at the molecular level
A mixture with large particles that settle out over time
A heterogeneous mixture where particles are intermediate in size between those in a solution and a suspension
A solid with a regular repeating structure