What is the common name for the hydrated form of Copper(II) Sulfate?
Epsom salt
Gypsum
Blue vitriol
Table salt
Copper(II) Sulfate (CuSO4) is a versatile chemical compound known for its vibrant blue color. In chemistry, it’s recognized for its role in various applications, including agriculture, where it acts as a fungicide, and in copper refining, serving as an essential electrolyte. Copper(II) sulfate is highly valued in educational chemistry for showcasing crystal growth and investigating transition metals. Its vibrant blue crystals offer a visual and hands-on way to learn about crystallization and chemical properties. This compound enriches students’ chemistry knowledge and sparks interest in science through engaging experiments, making it a key resource for understanding chemistry fundamentals. Its effectiveness in water treatment processes, by combating algae and microbial contaminants, underscores its importance across multiple industries.
| Property | Value |
|---|---|
| Formula | CuSO₄ |
| Hill formula | CuO₄S |
| Name | Copper(II) sulfate |
| IUPAC name | Copper sulfate |
| Alternate names | Cupric sulfate |
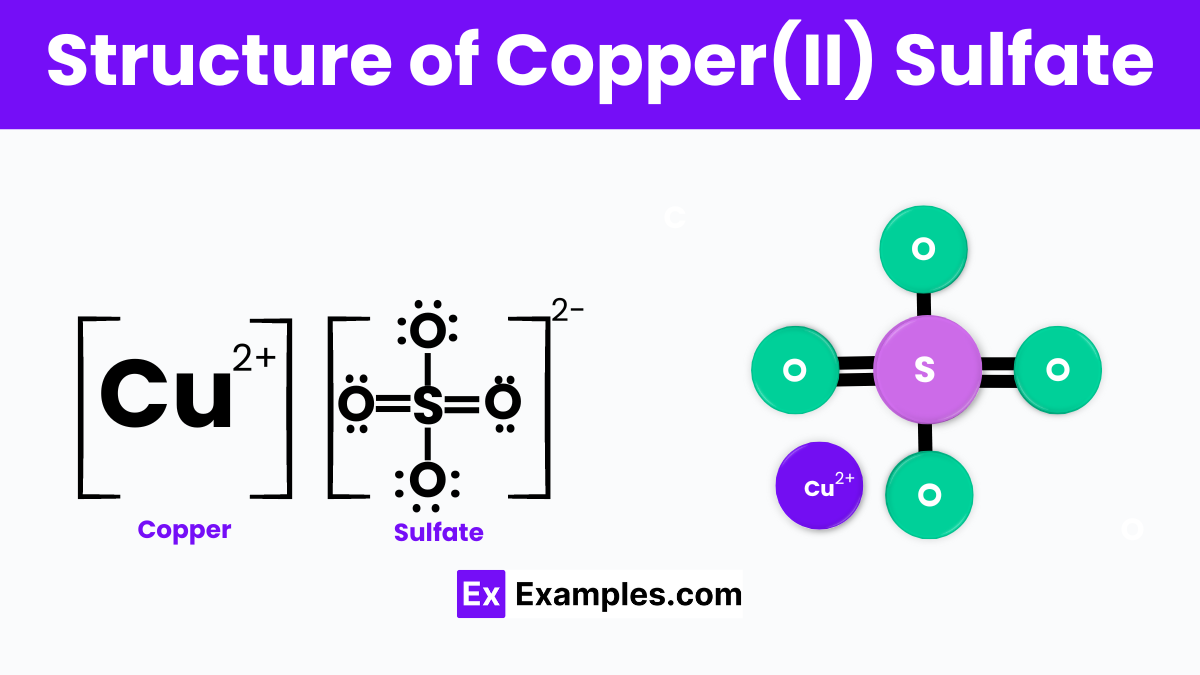
The structure of Copper(II) Sulfate, specifically in its anhydrous form (CuSO₄), features a central copper ion (Cu²⁺) connected to four sulfate (SO₄²⁻) ions arranged in a tetrahedral geometry. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure.
In its more common hydrated form, Copper(II) Sulfate Pentahydrate (CuSO₄·5H₂O), the structure becomes more complex. The copper ion is still at the center, but now it’s surrounded by four water molecules and one sulfate ion in a distorted square pyramid shape. The fifth water molecule is not directly bonded to the copper ion but is attached through hydrogen bonding to the sulfate ion and other water molecules, stabilizing the structure and contributing to the compound’s distinctive bright blue crystals.These structural properties of Copper(II) Sulfate are crucial for its chemical behavior, including its solubility in water, its ability to form complexes, and its use in various chemical reactions and applications.
Copper(II) sulfate, known for its vivid blue color in its hydrated form, is a versatile chemical compound with a wide range of applications. The preparation of Copper(II) sulfate involves a simple reaction between copper(II) oxide (CuO) and sulfuric acid (H₂SO₄) in an aqueous solution. To begin, approximately 100 ml of distilled water is added to a clean beaker, followed by the gradual addition of about 5 grams of copper(II) oxide. The mixture is then carefully treated with sulfuric acid, added dropwise while stirring, to ensure complete dissolution of the copper oxide. The reaction that takes place is represented by the equation:
This reaction illustrates the transformation of copper(II) oxide and sulfuric acid into Copper(II) sulfate and water, signifying the formation of Copper(II) sulfate in the aqueous solution.
Upon the addition of sulfuric acid, the solution is gently heated and continuously stirred to facilitate the reaction. As the copper(II) oxide dissolves, the solution adopts a characteristic blue color, indicative of Copper(II) sulfate formation. To make a clear blue solution of Copper(II) Sulfate, heat the solution, cool it to room temperature, filter out solids, and evaporate water for crystals.
The entire process, from the dissolution of copper(II) oxide to the crystallization of Copper(II) sulfate, is not only a demonstration of basic chemical reactions but also highlights the preparation of a compound with significant practical applications. Safety precautions, including the use of protective gear and handling chemicals with care, are paramount throughout the experiment to ensure a safe and effective outcome. Copper(II) sulfate, with its wide range of uses, from agriculture to education, exemplifies the importance of chemical synthesis in producing valuable compounds.
| Property | Description |
|---|---|
| Appearance | Bright blue, crystalline solid |
| Molecular Weight | 249.68 g/mol (Pentahydrate) |
| Density | 2.286 g/cm³ (Pentahydrate) |
| Melting Point | 110°C (230°F; decomposes to anhydrous form) |
| Boiling Point | Decomposes before boiling |
| Solubility in Water | 31.6 g/100 mL (0°C); 203.3 g/100 mL (100°C) |
| Chemical Formula | CuSO4·5H2O (Pentahydrate) |
| pH | Acidic (around 4.5 for a 5% solution at 25°C) |
Copper(II) Sulfate readily dissolves in water, forming a blue aqueous solution. The anhydrous form can absorb moisture from the air, transitioning to the hydrated form, Copper(II) Sulfate Pentahydrate (CuSO₄·5H₂O), which is known for its bright blue crystals.
In aqueous solutions, Copper(II) Sulfate exhibits acidic properties due to the release of H+ ions from the sulfate ion (SO₄²⁻). It can react with bases to form copper-containing salts.
Copper(II) Sulfate can form complexes with other ions or molecules. For example, when mixed with ammonia (NH₃), it forms a deep blue complex, [Cu(NH₃)4]²⁺, showcasing its ability to undergo ligand exchange reactions.
Being a good oxidizing agent, Copper(II) Sulfate can participate in redox (reduction-oxidation) reactions. Copper(II) Sulfate can oxidize metals, such as turning iron into Iron(II) Sulfate while releasing copper metal in the process.
Upon heating, Copper(II) Sulfate Pentahydrate loses its water of hydration, turning into the anhydrous form, which is white. Further heating can decompose it into Copper(II) Oxide (CuO), sulfur dioxide (SO₂), and oxygen (O₂).
Copper(II) Sulfate reacts with strong acids, such as concentrated hydrochloric acid, to form Copper(II) Chloride (CuCl₂) and sulfuric acid. With bases, it forms copper hydroxide precipitates.
| Property | Value |
|---|---|
| CAS Registry Number | 7758-98-7 |
| PubChem Compound ID | 24462 |
| SMILES Identifier | [O-]S(=O)(=O)[O-].[Cu+2] |
| InChI Identifier | InChI=1/Cu.H2O4S/c;1-5(2, 3)4/h;(H2,1,2,3,4)/q+2;/p-2/fCu.O4S/qm;-2 |
| RTECS Number | GL8800000 |
| MDL Number | MFCD00010981 |
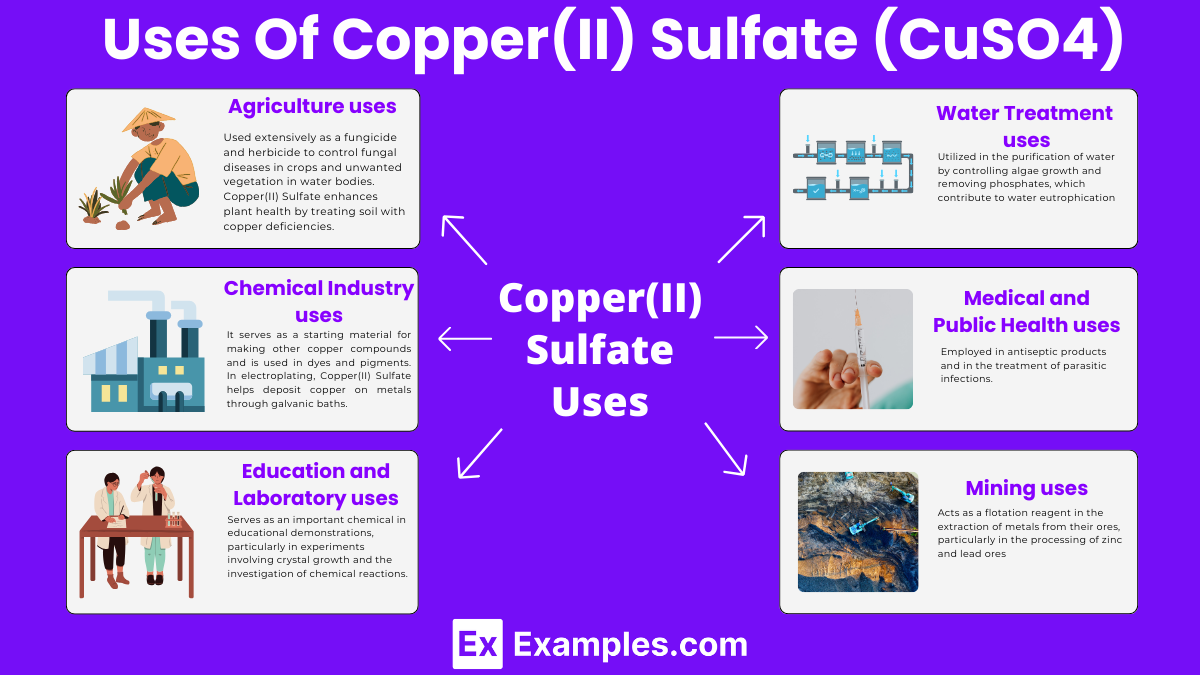
Used extensively as a fungicide and herbicide to control fungal diseases in crops and unwanted vegetation in water bodies. Copper(II) Sulfate enhances plant health by treating soil with copper deficiencies.
It serves as a starting material for making other copper compounds and is used in dyes and pigments. In electroplating, Copper(II) Sulfate helps deposit copper on metals through galvanic baths.
Serves as an important chemical in educational demonstrations, particularly in experiments involving crystal growth and the investigation of chemical reactions. In laboratories, Copper(II) Sulfate serves as a reagent in many analytical methods..
Utilized in the purification of water by controlling algae growth and removing phosphates, which contribute to water eutrophication. Its antibacterial properties help in preventing the spread of waterborne diseases.
Employed in antiseptic products and in the treatment of parasitic infections.
Used in traditional patination processes to impart aged appearances to copper artworks and sculptures.
Acts as a flotation reagent in the extraction of metals from their ores, particularly in the processing of zinc and lead ores.
It plays a crucial role in preventing and treating fungal diseases in crops, ensuring healthier plant growth and improved crop yields. By correcting copper deficiencies in the soil, it supports the overall nutritional health of plants.
Its use in water treatment processes helps in controlling algae blooms and removing microbes, leading to cleaner, safer water bodies. This contributes to the maintenance of ecosystems and supports public health by reducing waterborne diseases.
In the chemical industry, Copper(II) Sulfate is crucial for making various copper compounds, electroplating, and producing pigments. These applications are essential for manufacturing processes across various sectors.
The compound’s antifungal and antibacterial properties make it effective in preventing hoof diseases in cattle and other livestock, promoting animal health and reducing the risk of disease spread.
By controlling unwanted vegetation and pests in aquatic environments, Copper(II) Sulfate helps in managing ecosystems and maintaining biodiversity.
Artists and craftsmen utilize Copper(II) Sulfate for patination processes, enabling the creation of aesthetically pleasing artworks with unique finishes.
Ingesting Copper(II) Sulfate can cause nausea, vomiting, and damage to body organs. Immediate medical attention is crucial.
Direct skin contact with Copper(II) Sulfate may cause irritation or allergic reactions.
The common name for Copper(II) Sulfate is Blue Vitriol, recognized for its bright blue color.
Copper(II) Sulfate (CuSO₄) is an ionic compound, consisting of copper cations (Cu²⁺) and sulfate anions (SO₄²⁻).
Aqueous Copper(II) Sulfate is a blue liquid formed when CuSO₄ dissolves in water, used in many chemical processes.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the common name for the hydrated form of Copper(II) Sulfate?
Epsom salt
Gypsum
Blue vitriol
Table salt
What color is anhydrous Copper(II) Sulfate?
Blue
White
Green
Red
What is the primary use of Copper(II) Sulfate in agriculture?
Pesticide
Fertilizer
Fungicide
Soil conditioner
What happens to hydrated Copper(II) Sulfate when it is heated?
It turns green
It turns blue
It loses water and turns white
It decomposes into sulfur dioxide and copper oxide
Which of the following is a use of Copper(II) Sulfate in the laboratory?
As a pH indicator
As a reagent for Benedict's test
As a primary standard for titration
As a catalyst in esterification reactions
What is the molar mass of Copper(II) Sulfate (CuSO₄)?
159.6 g/mol
169.6 g/mol
179.6 g/mol
189.6 g/mol
How many water molecules are associated with each formula unit in hydrated Copper(II) Sulfate?
2
3
4
5
What is the oxidation state of copper in Copper(II) Sulfate?
0
+1
+2
+3
Which of the following is a common method for preparing Copper(II) Sulfate in the laboratory?
Reacting copper metal with sulfuric acid
Reacting copper oxide with hydrochloric acid
Reacting copper carbonate with nitric acid
Reacting copper nitrate with sulfuric acid
What is the role of Copper(II) Sulfate in the Fehling's solution?
It acts as an oxidizing agent
It acts as a reducing agent
It acts as a catalyst
It acts as a solvent
Before you leave, take our quick quiz to enhance your learning!

