What is the chemical symbol for curium?
Cm
Cu
Cr
Cf
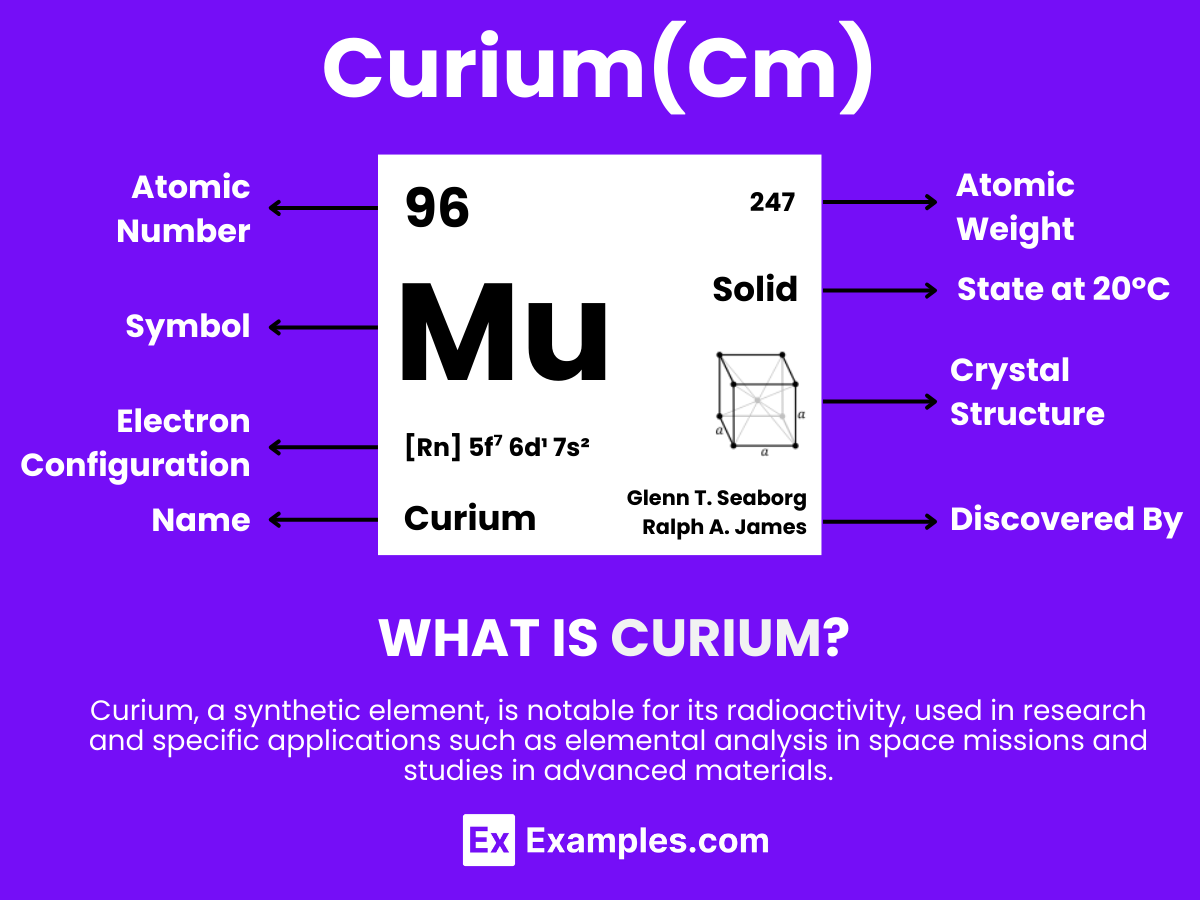
Curium, a synthetic element with the symbol Cm and atomic number 96, stands out in the periodic table for its remarkable properties and applications. This complete guide delves into the world of Curium, offering insights into its discovery, characteristics, and significant role in scientific advancements. Through practical examples, we explore its uses in various fields, from medicine to space exploration, highlighting Curium’s impact on technology and research. Discover the fascinating aspects of Curium, from its complex compounds to its contribution to nuclear science.
Curium is a synthetic, radioactive element that does not occur naturally on Earth. It is part of the actinide series in the periodic table and has the symbol Cm with the atomic number 96. Created in laboratories through the bombardment of plutonium with alpha particles, Curium is named after Marie and Pierre Curie, pioneers in the field of radioactivity. This element is known for its high radioactivity and has applications in scientific research, specifically in areas like nuclear reactors and certain types of medical equipment.
| Actinium | Berkelium |
| Thorium | Californium |
| Protactinium | Einsteinium |
| Uranium | Fermium |
| Neptunium | Mendelevium |
| Plutonium | Nobelium |
| Americium | Lawrencium |
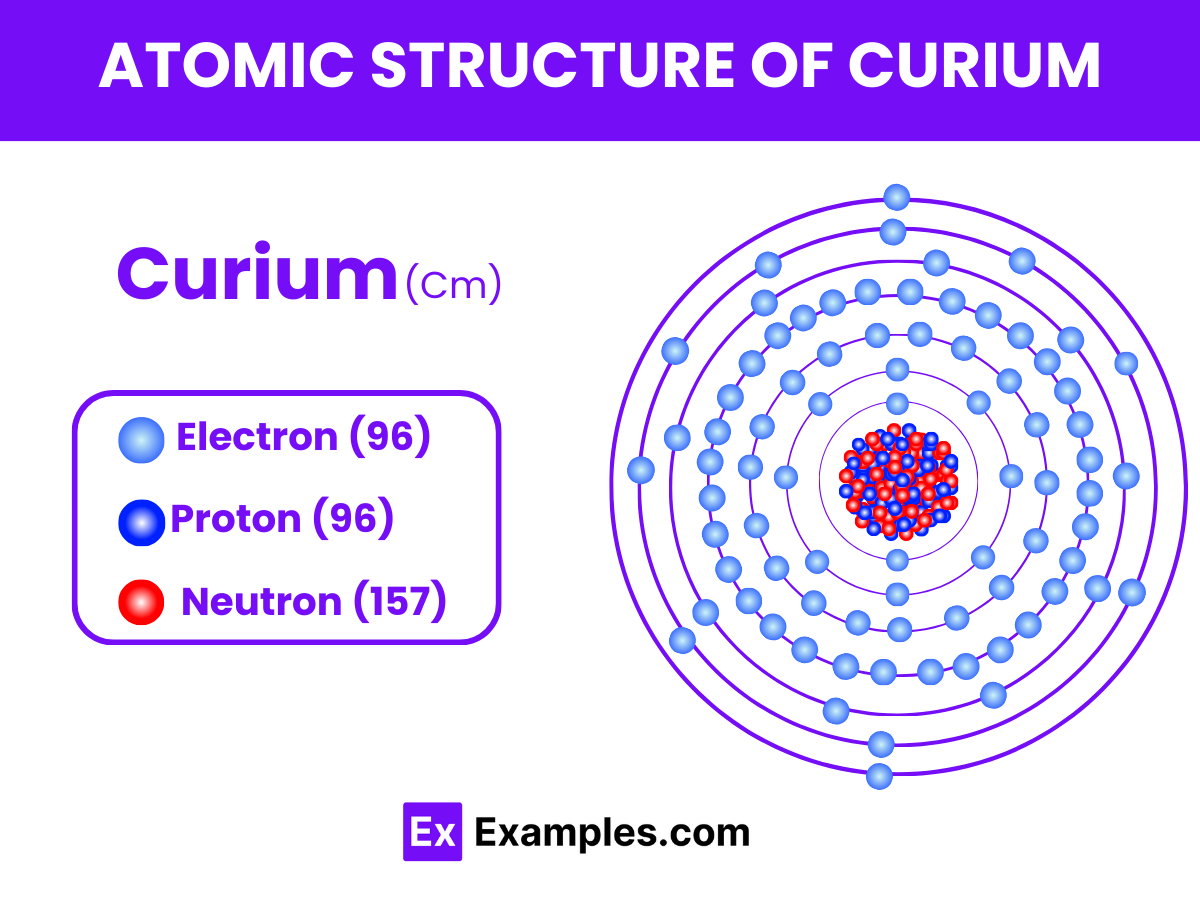
Curium, symbolized as Cm, is a synthetic element with an atomic number of 96 in the periodic table. As a member of the actinide series, curium shares common features with other actinides, including a complex electron configuration and pronounced radioactivity. Here, we delve into the nuances of curium’s atomic structure, shedding light on its electron arrangement, nuclear composition, and the implications of its radioactive nature.
Electron Configuration: The electron configuration of curium is [Rn] 5f⁷ 6d¹ 7s². This configuration indicates that curium has electrons in the 5f, 6d, and 7s orbitals, following the actinides’ characteristic of filling the 5f orbital. The presence of electrons in the 5f orbital is key to the element’s chemical behavior and its categorization as a part of the f-block elements in the periodic table.
Nucleus Composition: Curium’s nucleus comprises protons and neutrons, with the number of protons defining its place as element 96. The isotope of curium most commonly used and studied is curium-244, which contains 148 neutrons in addition to its 96 protons. The nucleus of curium isotopes is unstable, leading to radioactive decay through alpha emission, which is a hallmark of heavy, transuranic elements.
Radioactive Properties: The atomic structure of curium underpins its radioactive nature. Curium isotopes, including curium-242, curium-244, and several others, exhibit varying half-lives, from 162.8 days for curium-242 to 18.1 million years for curium-247. The radioactivity of curium is primarily due to its alpha decay, where the nucleus emits an alpha particle (two protons and two neutrons), transforming into an atom of a different element with a lower atomic number.
Applications and Implications: The radioactive properties of curium, stemming from its atomic structure, have practical applications in areas such as space exploration, where curium isotopes serve as a compact and reliable power source for spacecraft. Additionally, the study of curium’s atomic structure and its behavior under various conditions contributes to the field of nuclear chemistry, enhancing our understanding of nuclear reactions, stability, and the synthesis of new elements.
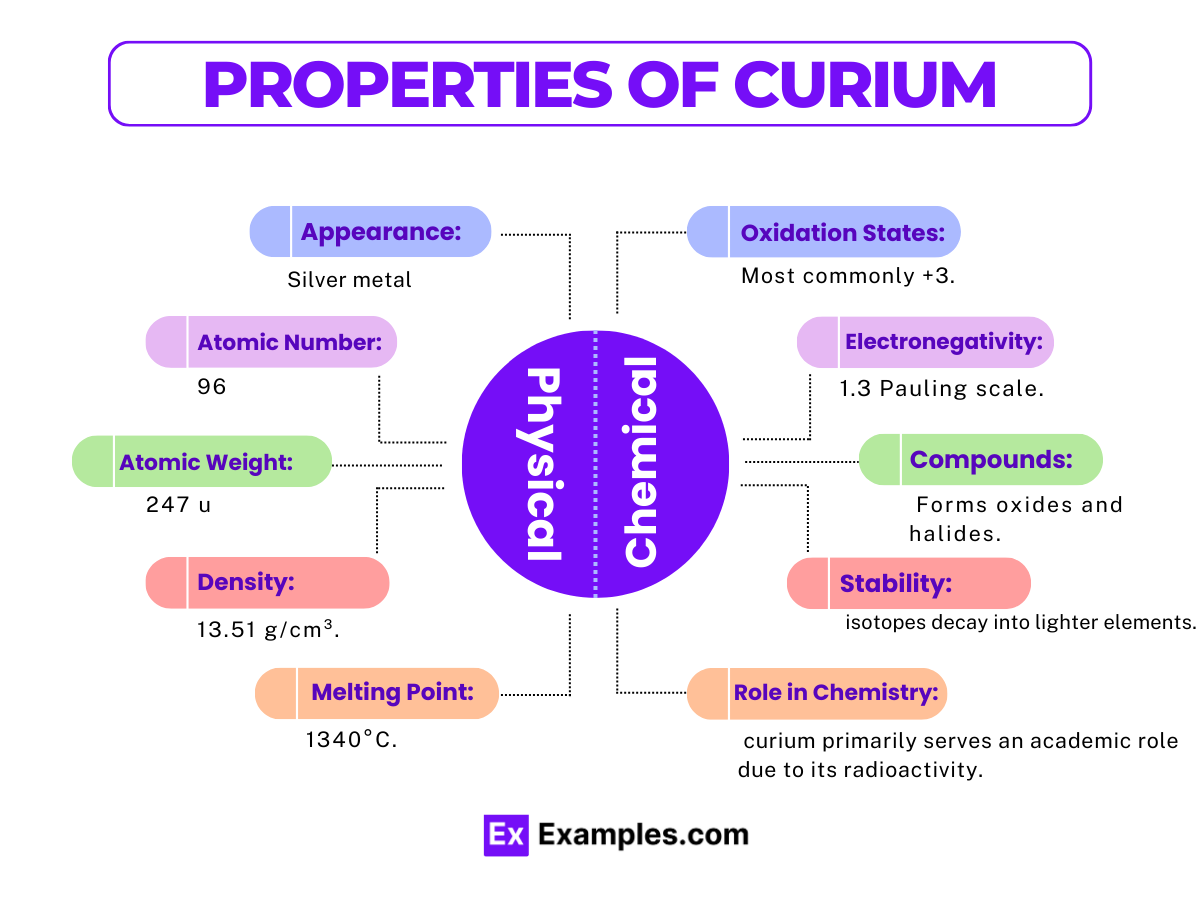
| Property | Description |
|---|---|
| Atomic Number | 96 |
| Symbol | Cm |
| Atomic Weight | 247 amu (for Curium-247, one of its most stable isotopes) |
| Density | Approximately 13.51 g/cm³ at room temperature |
| Melting Point | 1340°C (2444°F) |
| Boiling Point | 3110°C (5630°F) |
| State at Room Temperature | Solid |
| Color | Silvery metallic |
| Radioactivity | Highly radioactive, with multiple isotopes emitting alpha particles |
| Crystal Structure | Hexagonal close-packed (hcp) at room temperature; transforms into a face-centered cubic (fcc) structure at higher temperatures |
| Conductivity | Good thermal conductor |
| Electronegativity | 1.3 (Pauling scale) |
| Heat of Fusion | 15 kJ/mol |
| Heat of Vaporization | 400 kJ/mol |
Curium is a synthetic, radioactive element with the symbol Cm and atomic number 96. Its chemical properties are characterized by its reactivity and the ability to form various compounds and complexes due to its multiple oxidation states, primarily +3 and +4.
Key Properties:
| Property | Value | Units |
|---|---|---|
| Standard Atomic Weight | 247 (for Curium-247, its most stable isotope) | g/mol |
| Melting Point | 1340 | °C |
| Boiling Point | 3110 | °C |
| Density at Room Temperature | 13.51 | g/cm³ |
| Heat of Fusion | 15 | kJ/mol |
| Heat of Vaporization | 400 | kJ/mol |
| Specific Heat Capacity | – | J/(kg·K) |
| Thermal Conductivity | – | W/(m·K) |
| Thermal Expansion | – | /K |
| Property | Description |
|---|---|
| State at Room Temperature | Solid |
| Color | Silvery metallic |
| Crystal Structure | Hexagonal close-packed (hcp) at room temperature; transforms into a face-centered cubic (fcc) structure at higher temperatures |
| Radioactivity | Highly radioactive, primarily alpha emission |
| Hardness | – |
| Magnetic Properties | Paramagnetic |
| Solubility | Insoluble in water, soluble in acids |
| Property | Description |
|---|---|
| Electrical Conductivity | Low; behaves similarly to other actinide metals, with conductivity decreasing with temperature |
| Magnetic Properties | Paramagnetic; some isotopes exhibit antiferromagnetic properties at low temperatures |
| Electric Resistivity | High; increases with temperature, indicative of metallic behavior |
| Property | Description |
|---|---|
| Isotopes | Numerous; including Cm-242 to Cm-248, with varying stability and half-lives |
| Most Stable Isotopes | Cm-247 (half-life of 15.6 million years) and Cm-248 (half-life of 348,000 years) |
| Primary Decay Modes | Alpha emission, spontaneous fission (for heavier isotopes) |
| Neutron Cross Section | High; makes it valuable for use in neutron capture reactions in nuclear reactors |
| Radioactivity | Highly radioactive; requires careful handling and shielding |
| Role in Nuclear Reactions | Can be used as a fuel in nuclear reactors and for producing higher actinides and transactinide elements |
Curium is a synthetic element that does not occur naturally. It is produced in nuclear reactors through the neutron bombardment of heavier elements, typically plutonium or americium. The process of preparing curium involves several steps, including neutron irradiation, chemical separation, and purification processes. Below is an overview of the general procedure used to prepare curium:
The final product is a sample of curium in the desired form, typically as a compound. Due to its high radioactivity, handling and storage of curium require specialized facilities to protect researchers and the environment from radiation exposure.
The preparation of curium is a complex process that requires sophisticated equipment and expertise in nuclear chemistry. The ability to produce and isolate curium has facilitated its use in scientific research, particularly in the study of transuranic elements and their properties.
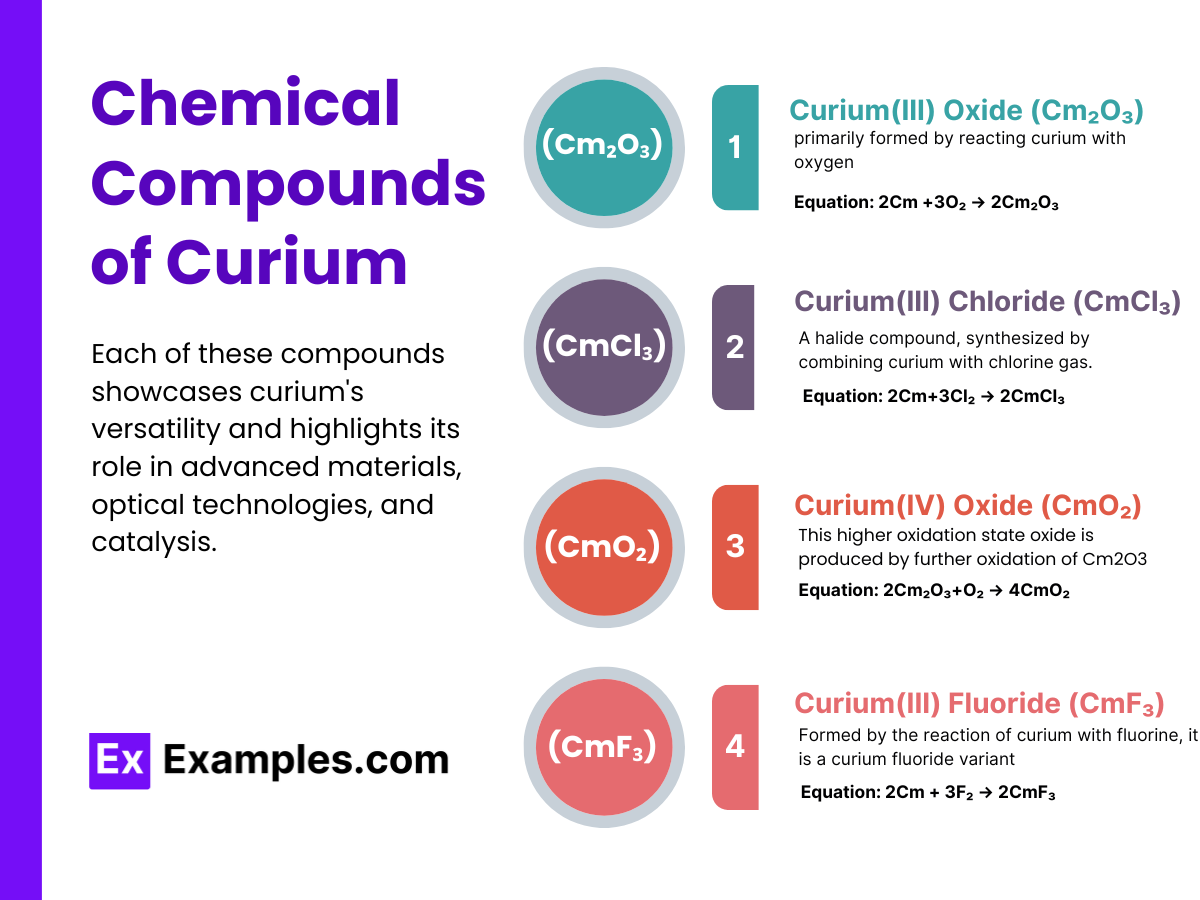
A stable oxide of curium, primarily formed by reacting curium with oxygen.
Equation: 2Cm +3O₂ → 2Cm₂O₃
A halide compound, synthesized by combining curium with chlorine gas.
Equation: 2Cm+3Cl₂ → 2CmCl₃
This higher oxidation state oxide is produced by further oxidation of Cm2O3.
Equation: 2Cm₂O₃+O₂ → 4CmO₂
Formed by the reaction of curium with fluorine, it is a curium fluoride variant.
Equation: 2Cm + 3F₂ → 2CmF₃
A bromide compound of curium, obtained by reacting it with bromine.
Equation: 2Cm + 3Br₂ → 2CmBr₃
This iodide variant is synthesized through the direct reaction of curium with iodine.
Equation: 2Cm + 3I₂ → 2CmI₃
| Isotope | Half-Life | Decay Mode | Primary Use/Application |
|---|---|---|---|
| Cm-242 | 162.8 days | Alpha decay | Heat source in space missions |
| Cm-243 | 29.1 years | Alpha decay | Target for producing heavier elements |
| Cm-244 | 18.1 years | Alpha decay, spontaneous fission | Heat source, neutron source |
| Cm-245 | 8,500 years | Alpha decay | Target for producing transcurium elements |
| Cm-246 | 4,760 years | Alpha decay, spontaneous fission | Research in nuclear science |
| Cm-247 | 15.6 million years | Alpha decay | Studies of nuclear reactions and processes |
| Cm-248 | 348,000 years | Alpha decay, spontaneous fission | Research in nuclear science, potential for dating |
| Cm-250 | 8,300 years | Spontaneous fission, alpha decay | Research, potential as a neutron source |
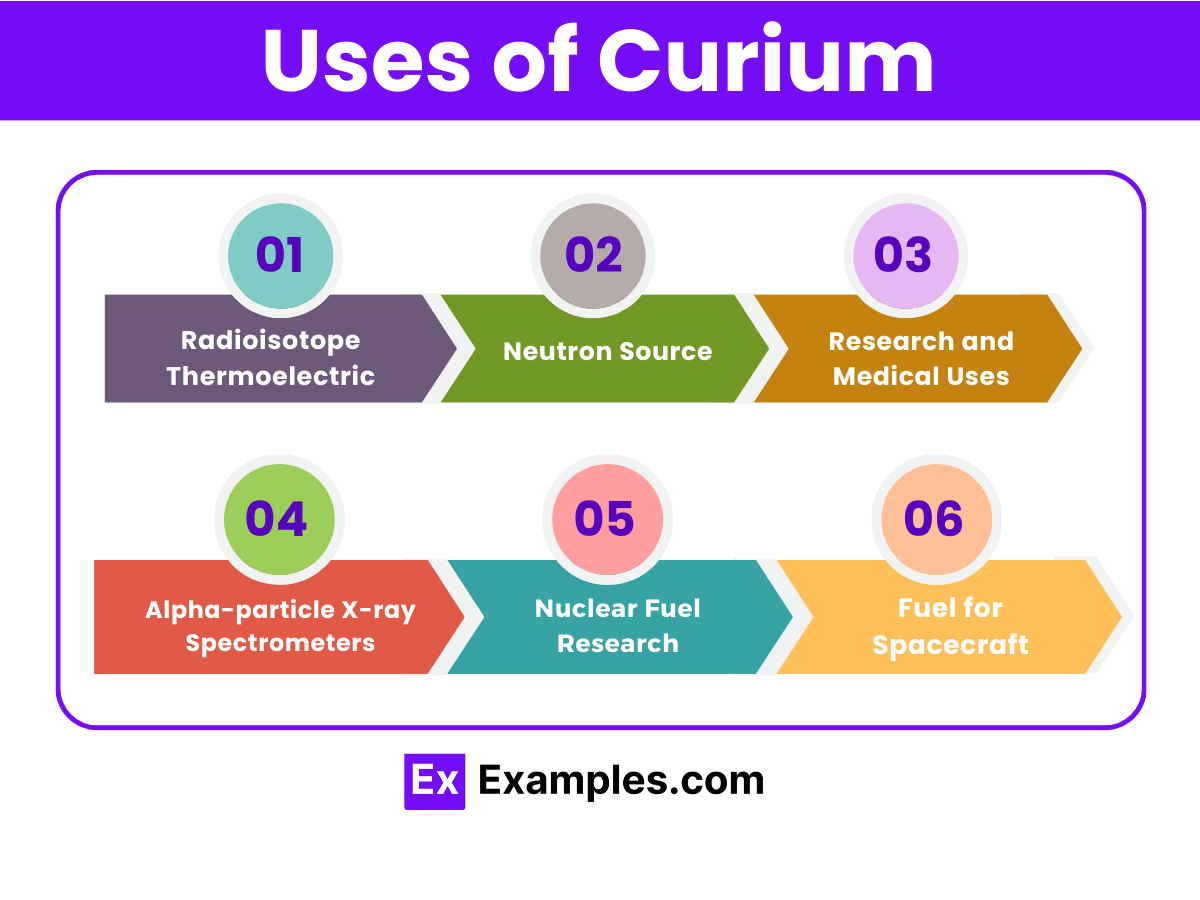
Radioisotope Thermoelectric Generators (RTGs)
Neutron Source
Research and Medical Uses
Alpha-particle X-ray Spectrometers
Nuclear Fuel Research
Fuel for Spacecraft: Considering its potential as a power source in RTGs, curium could provide the energy necessary for long-duration space missions beyond the solar system or to shadowed regions of planets and moons where solar power is
Curium is not found naturally and is produced in minute quantities in nuclear reactors. The typical production method involves:
Despite its radioactivity, Curium finds use in several specialized fields, leveraging its unique properties for beneficial purposes:
The production and use of Curium are constrained by its radioactivity, necessitating strict safety protocols to protect researchers, workers, and the environment. The specialized applications of Curium underscore the broader scientific and technological interest in synthetic elements and their potential to contribute to advanced technological solutions, from space exploration to medical treatments.

Curium, a synthetic element with the symbol Cm and atomic number 96, stands out in the periodic table for its remarkable properties and applications. This complete guide delves into the world of Curium, offering insights into its discovery, characteristics, and significant role in scientific advancements. Through practical examples, we explore its uses in various fields, from medicine to space exploration, highlighting Curium’s impact on technology and research. Discover the fascinating aspects of Curium, from its complex compounds to its contribution to nuclear science.
Curium is a synthetic, radioactive element that does not occur naturally on Earth. It is part of the actinide series in the periodic table and has the symbol Cm with the atomic number 96. Created in laboratories through the bombardment of plutonium with alpha particles, Curium is named after Marie and Pierre Curie, pioneers in the field of radioactivity. This element is known for its high radioactivity and has applications in scientific research, specifically in areas like nuclear reactors and certain types of medical equipment.
Formula: Cm
Composition: Curium is composed entirely of curium atoms, categorizing it as an elemental substance.
Bond Type: Being an element, curium in its pure form does not engage in bonding. It is capable of forming diverse chemical bonds, such as ionic and covalent, with other elements. This characteristic enables curium to participate in the formation of a variety of compounds.
Molecular Structure: Curium, in its elemental state, does not have a molecular structure. It possesses a metallic structure, typically manifesting in a face-centered cubic crystalline form. This structure reflects curium’s nature as a dense, silvery metal, which may exhibit a slight golden hue.
Electron Sharing: Curium can share electrons to establish covalent bonds or donate electrons to create ionic bonds. In its compounds, curium often appears in a +3 oxidation state (Cm³⁺), though it can exhibit other oxidation states. This versatility underscores curium’s ability to form a wide range of chemical species.
Significance: Curium plays a significant role across various fields, notably in nuclear research and applications. Its isotopes, particularly curium-244 and curium-242, are used as power sources in space exploration and in medical devices for pacemakers. Curium’s ability to emit alpha particles is exploited in alpha-particle X-ray spectrometers, instruments aboard Mars rovers for analyzing soil and rock samples. The element’s radioactive properties and its use in targeted alpha therapy highlight its importance in both scientific exploration and medical treatments.
Role in Chemistry: The chemical properties of curium, a member of the actinide series, attract attention in chemical research, emphasizing the unique characteristics of transuranic elements. Its compounds demonstrate significant radioactivity, which is critical for understanding nuclear reactions and decay processes. Curium’s behavior in compounds and its interactions with other elements are crucial for advancing nuclear chemistry and developing new materials for radiation detection and nuclear fuel applications. The study of curium and its compounds enhances our grasp of actinide chemistry, contributing to the development of nuclear science and technology.

Curium, symbolized as Cm, is a synthetic element with an atomic number of 96 in the periodic table. As a member of the actinide series, curium shares common features with other actinides, including a complex electron configuration and pronounced radioactivity. Here, we delve into the nuances of curium’s atomic structure, shedding light on its electron arrangement, nuclear composition, and the implications of its radioactive nature.
Electron Configuration: The electron configuration of curium is [Rn] 5f⁷ 6d¹ 7s². This configuration indicates that curium has electrons in the 5f, 6d, and 7s orbitals, following the actinides’ characteristic of filling the 5f orbital. The presence of electrons in the 5f orbital is key to the element’s chemical behavior and its categorization as a part of the f-block elements in the periodic table.
Nucleus Composition: Curium’s nucleus comprises protons and neutrons, with the number of protons defining its place as element 96. The isotope of curium most commonly used and studied is curium-244, which contains 148 neutrons in addition to its 96 protons. The nucleus of curium isotopes is unstable, leading to radioactive decay through alpha emission, which is a hallmark of heavy, transuranic elements.
Radioactive Properties: The atomic structure of curium underpins its radioactive nature. Curium isotopes, including curium-242, curium-244, and several others, exhibit varying half-lives, from 162.8 days for curium-242 to 18.1 million years for curium-247. The radioactivity of curium is primarily due to its alpha decay, where the nucleus emits an alpha particle (two protons and two neutrons), transforming into an atom of a different element with a lower atomic number.
Applications and Implications: The radioactive properties of curium, stemming from its atomic structure, have practical applications in areas such as space exploration, where curium isotopes serve as a compact and reliable power source for spacecraft. Additionally, the study of curium’s atomic structure and its behavior under various conditions contributes to the field of nuclear chemistry, enhancing our understanding of nuclear reactions, stability, and the synthesis of new elements.

Property | Description |
|---|---|
Atomic Number | 96 |
Symbol | Cm |
Atomic Weight | 247 amu (for Curium-247, one of its most stable isotopes) |
Density | Approximately 13.51 g/cm³ at room temperature |
Melting Point | 1340°C (2444°F) |
Boiling Point | 3110°C (5630°F) |
State at Room Temperature | Solid |
Color | Silvery metallic |
Radioactivity | Highly radioactive, with multiple isotopes emitting alpha particles |
Crystal Structure | Hexagonal close-packed (hcp) at room temperature; transforms into a face-centered cubic (fcc) structure at higher temperatures |
Conductivity | Good thermal conductor |
Electronegativity | 1.3 (Pauling scale) |
Heat of Fusion | 15 kJ/mol |
Heat of Vaporization | 400 kJ/mol |
Curium is a synthetic, radioactive element with the symbol Cm and atomic number 96. Its chemical properties are characterized by its reactivity and the ability to form various compounds and complexes due to its multiple oxidation states, primarily +3 and +4.
Key Properties:
Reactivity: Curium tarnishes in air, reacting with oxygen to form oxides. It also reacts with halogens to produce halides.
Oxidation States: The +3 oxidation state is more stable and common, leading to compounds like Curium(III) oxide (Cm₂ O₃ ) and Curium(III) fluoride (CmF₃ ). Curium can also exhibit a +4 state, forming Curium(IV) oxide (CmO₂ ).
Complex Formation: Curium forms complexes with various ligands, useful in nuclear medicine and environmental remediation.
Radiochemical Behavior: Its radioactivity affects the chemical stability of curium compounds and requires special handling.
Property | Value | Units |
|---|---|---|
Standard Atomic Weight | 247 (for Curium-247, its most stable isotope) | g/mol |
Melting Point | 1340 | °C |
Boiling Point | 3110 | °C |
Density at Room Temperature | 13.51 | g/cm³ |
Heat of Fusion | 15 | kJ/mol |
Heat of Vaporization | 400 | kJ/mol |
Specific Heat Capacity | – | J/(kg·K) |
Thermal Conductivity | – | W/(m·K) |
Thermal Expansion | – | /K |
Property | Description |
|---|---|
State at Room Temperature | Solid |
Color | Silvery metallic |
Crystal Structure | Hexagonal close-packed (hcp) at room temperature; transforms into a face-centered cubic (fcc) structure at higher temperatures |
Radioactivity | Highly radioactive, primarily alpha emission |
Hardness | – |
Magnetic Properties | Paramagnetic |
Solubility | Insoluble in water, soluble in acids |
Property | Description |
|---|---|
Electrical Conductivity | Low; behaves similarly to other actinide metals, with conductivity decreasing with temperature |
Magnetic Properties | Paramagnetic; some isotopes exhibit antiferromagnetic properties at low temperatures |
Electric Resistivity | High; increases with temperature, indicative of metallic behavior |
Property | Description |
|---|---|
Isotopes | Numerous; including Cm-242 to Cm-248, with varying stability and half-lives |
Most Stable Isotopes | Cm-247 (half-life of 15.6 million years) and Cm-248 (half-life of 348,000 years) |
Primary Decay Modes | Alpha emission, spontaneous fission (for heavier isotopes) |
Neutron Cross Section | High; makes it valuable for use in neutron capture reactions in nuclear reactors |
Radioactivity | Highly radioactive; requires careful handling and shielding |
Role in Nuclear Reactions | Can be used as a fuel in nuclear reactors and for producing higher actinides and transactinide elements |
Curium is a synthetic element that does not occur naturally. It is produced in nuclear reactors through the neutron bombardment of heavier elements, typically plutonium or americium. The process of preparing curium involves several steps, including neutron irradiation, chemical separation, and purification processes. Below is an overview of the general procedure used to prepare curium:
Starting Material: The process begins with a target material, usually plutonium (Pu) or americium (Am), placed inside a nuclear reactor.
Irradiation: The target material is exposed to a flux of neutrons in the reactor. The neutrons are absorbed by the nuclei of the target atoms, increasing their atomic number.
Plutonium-239, for example, can capture neutrons to form Plutonium-240, which further captures neutrons to eventually form Curium-242 through a series of beta decays: Cm₂₄₂Pu²³⁹(n,γ)Pu²⁴⁰(n,γ)Pu²⁴¹(β⁻)Am²⁴¹(n,γ)Am²⁴²m(β⁻)Cm²⁴²
Cooling: After irradiation, the target material is allowed to cool, decreasing its radioactivity to safer levels.
Dissolution: The irradiated material is then dissolved in a suitable acid, such as nitric acid, to form a solution containing a mixture of different elements and isotopes.
Separation: Chemical separation techniques, such as ion exchange or solvent extraction, are employed to isolate curium from other elements. For instance, the PUREX (Plutonium-Uranium Extraction) process can be adapted for separating curium.
Further Separation: Additional steps, such as further ion exchange or precipitation reactions, may be necessary to purify the curium from any remaining contaminants.
Conversion: The purified curium is often converted into a specific compound, such as curium oxide (CmO₂ ) or curium chloride (CmCl₃ ), depending on its intended use.
The final product is a sample of curium in the desired form, typically as a compound. Due to its high radioactivity, handling and storage of curium require specialized facilities to protect researchers and the environment from radiation exposure.
The preparation of curium is a complex process that requires sophisticated equipment and expertise in nuclear chemistry. The ability to produce and isolate curium has facilitated its use in scientific research, particularly in the study of transuranic elements and their properties.

A stable oxide of curium, primarily formed by reacting curium with oxygen.
Equation: 2Cm +3O₂ → 2Cm₂O₃
A halide compound, synthesized by combining curium with chlorine gas.
Equation: 2Cm+3Cl₂ → 2CmCl₃
This higher oxidation state oxide is produced by further oxidation of Cm2O3.
Equation: 2Cm₂O₃+O₂ → 4CmO₂
Formed by the reaction of curium with fluorine, it is a curium fluoride variant.
Equation: 2Cm + 3F₂ → 2CmF₃
A bromide compound of curium, obtained by reacting it with bromine.
Equation: 2Cm + 3Br₂ → 2CmBr₃
This iodide variant is synthesized through the direct reaction of curium with iodine.
Equation: 2Cm + 3I₂ → 2CmI₃
Isotope | Half-Life | Decay Mode | Primary Use/Application |
|---|---|---|---|
Cm-242 | 162.8 days | Alpha decay | Heat source in space missions |
Cm-243 | 29.1 years | Alpha decay | Target for producing heavier elements |
Cm-244 | 18.1 years | Alpha decay, spontaneous fission | Heat source, neutron source |
Cm-245 | 8,500 years | Alpha decay | Target for producing transcurium elements |
Cm-246 | 4,760 years | Alpha decay, spontaneous fission | Research in nuclear science |
Cm-247 | 15.6 million years | Alpha decay | Studies of nuclear reactions and processes |
Cm-248 | 348,000 years | Alpha decay, spontaneous fission | Research in nuclear science, potential for dating |
Cm-250 | 8,300 years | Spontaneous fission, alpha decay | Research, potential as a neutron source |

Radioisotope Thermoelectric Generators (RTGs)
Curium-244, with its significant heat generation from radioactive decay, is used in RTGs to provide power for spacecraft, satellites, and remote terrestrial applications. The heat released by its decay is converted into electricity, offering a reliable power source for missions that extend beyond the reach of solar energy.
Neutron Source
The alpha particles emitted by Curium isotopes, such as Curium-242 and Curium-244, can be used to generate neutrons when combined with a lightweight element like beryllium. These neutron sources have applications in neutron radiography, in initiating nuclear reactions, and in oil well logging to measure the porosity of rocks.
Research and Medical Uses
Curium isotopes are used in scientific research to study the properties of actinides and in the development of new materials. In medicine, Curium-242 has been explored as a potential source for cancer treatment through neutron capture therapy, although its use is highly specialized due to the challenges of handling and the risks associated with radiation.
Alpha-particle X-ray Spectrometers
Instruments equipped with Curium sources, specifically Curium-244, have been deployed on planetary missions, such as the Mars rovers, to analyze the composition of rocks and soil. These spectrometers use the alpha particles and X-rays generated by the decay of Curium to excite atoms in the sample and analyze the emitted X-rays, providing valuable data on the elemental composition of extraterrestrial materials.
Nuclear Fuel Research
Research into the use of Curium in nuclear fuel, particularly in mixed oxide (MOX) fuels for advanced nuclear reactors, is ongoing. While the practical application of Curium in commercial nuclear power generation is limited by its radioactivity and scarcity, it provides a valuable model for understanding the behavior of heavy actinides in reactor conditions.
Fuel for Spacecraft: Considering its potential as a power source in RTGs, curium could provide the energy necessary for long-duration space missions beyond the solar system or to shadowed regions of planets and moons where solar power is
Curium is not found naturally and is produced in minute quantities in nuclear reactors. The typical production method involves:
Neutron Bombardment: Plutonium (Pu) or Americium (Am) isotopes are subjected to intense neutron bombardment in a nuclear reactor. For example, Plutonium-239 captures neutrons to become Plutonium-240, which with further neutron capture and beta decay, forms Curium isotopes.
Chemical Separation: After irradiation, the target material contains a mixture of different actinides and fission products. Chemical separation processes, such as solvent extraction, are used to isolate Curium from these mixtures.
Despite its radioactivity, Curium finds use in several specialized fields, leveraging its unique properties for beneficial purposes:
Alpha-particle X-ray Spectrometers: Curium-244, due to its alpha radiation, serves as a source in alpha-particle X-ray spectrometers on unmanned space missions, such as the Mars rovers. These instruments help analyze the elemental composition of rocks and soils on other planets.
Radioisotope Thermoelectric Generators (RTGs): The heat generated from the radioactive decay of Curium isotopes, particularly Curium-244, can be converted into electricity by RTGs. This application is crucial for providing power to spacecraft, satellites, and remote devices where solar power is unfeasible.
Neutron Sources: When combined with a beryllium target, the alpha particles emitted by Curium isotopes like Curium-242 and Curium-244 can produce neutrons through (α,n) reactions. These neutron sources are valuable for initiating nuclear reactions, non-destructive testing, and in applications requiring portable neutron sources.
Medical Research: Research into the use of Curium isotopes for cancer treatment explores their potential in neutron therapy, where the neutrons produced by Curium can be directed to kill cancer cells. This application, however, is in experimental stages due to the complexity and safety concerns related to handling radioactive materials.
Scientific Research: Curium’s radioactive properties and complex electron configurations make it an interesting subject for scientific studies, particularly in understanding the behavior of actinides and in the synthesis of super heavy elements.
The production and use of Curium are constrained by its radioactivity, necessitating strict safety protocols to protect researchers, workers, and the environment. The specialized applications of Curium underscore the broader scientific and technological interest in synthetic elements and their potential to contribute to advanced technological solutions, from space exploration to medical treatments.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons (94)
Neutrons (150)
Protons (94)
What is the chemical symbol for curium?
Cm
Cu
Cr
Cf
What is the atomic number of curium?
94
95
96
97
In which year was curium first synthesized?
1939
1944
1950
1962
Curium belongs to which series of the periodic table?
Lanthanide
Actinide
Transition metal
Halogen
What is the primary use of curium?
Fuel in nuclear reactors
Medical imaging
Catalysts in chemical reactions
Production of smoke detectors
Curium is typically stored under:
Air
Water
Argon gas
Oil
Which method is commonly used to produce curium?
Neutron bombardment of plutonium
Electrolysis of curium salts
Reduction of curium oxide
Combustion of curium sulfide
Curium is primarily known for its:
Toxicity
Fluorescence
Radioactivity
Magnetism
What color does curium exhibit in its pure form?
Silver
Gold
Black
Gray
Which property makes curium challenging to handle?
Its magnetic properties
Its high melting point
Its radioactivity
Its density
Before you leave, take our quick quiz to enhance your learning!

