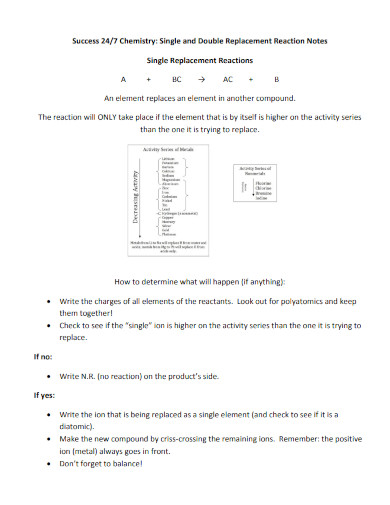
What is a double replacement reaction?
A reaction where two elements exchange places between two compounds
A reaction where a single compound breaks down into two or more elements or compounds
A reaction where two elements combine to form a single compound
A reaction where a compound reacts with oxygen to produce energy