What is the primary base metal in Duralumin?
Copper
Aluminum
Zinc
Nickel

Duralumin is a special kind of metal that is mostly made of aluminum, combined with a small amount of other metals like copper, magnesium, and manganese. This mix makes Duralumin stronger and harder than regular aluminum, but it still keeps its lightweight nature. It’s a favorite choice for making airplanes, boats, and even some sports equipment because it’s strong yet light, making things faster or easier to move. This metal was first developed in the early 20th century and has been an important material in many industries ever since.
Duralumin is a special kind of metal that’s mostly made of aluminum, but it also has a little bit of copper, manganese, and magnesium mixed in. Imagine it like a superhero team where aluminum is the leader because it makes up most of the team, but the other elements add their unique powers to make the team stronger. This mix makes duralumin really tough and light, perfect for making airplanes, spacecraft, and even some sports equipment. When scientists look at duralumin under a microscope, they see a structure where the aluminum atoms form the main part, but the tiny amounts of copper, manganese, and magnesium are sprinkled throughout, making the whole thing stronger and more resistant to damage.
To make duralumin, we start with aluminum, which is the main ingredient, and we mix it with small amounts of other metals like copper (about 3.5-4.5%), manganese (up to 0.5%), and magnesium (about 0.5-1%). It’s kind of like baking a cake where aluminum is the flour, but we also add some other ingredients to make it taste better. First, we melt the aluminum in a big furnace. When it’s all liquid, we add the copper, manganese, and magnesium. This mixture is then poured into molds and cooled down. After it solidifies, we have duralumin. But, there’s one more step! We heat the duralumin again at a lower temperature and then cool it down slowly. This process is called “aging,” and it makes the duralumin even stronger.
Here’s a simple way to look at the chemical mixture for making duralumin:
Remember, this isn’t a chemical reaction equation with exact ratios because the process is more about mixing and heat treatment than reacting. It’s the combination and the special treatment that give duralumin its strength.
| Property | Description |
|---|---|
| Color | Silvery-white, similar to aluminum but with a slightly duller finish due to the other metals mixed in. |
| Density | About 2.8 g/cm³, which means it’s pretty light, making it perfect for things that need to be strong but not heavy, like airplanes. |
| Strength | Stronger than pure aluminum, thanks to the added copper, manganese, and magnesium, which help make it tough and resistant to damage. |
| Ductility | It can be stretched into wires or bent into shapes without breaking, which is great for manufacturing. |
| Conductivity | Good electrical conductivity, though not as high as pure aluminum, making it useful in some electrical applications. |
| Corrosion Resistance | Has a good resistance to corrosion, especially when treated, which means it doesn’t rust or wear away easily. |
It is known for not rusting easily, due to aluminum’s ability to form a protective aluminum oxide layer when it comes into contact with air. This acts like an invisible shield, keeping the metal safe from environmental damage.
When duralumin comes into contact with strong acids, such as hydrochloric acid, it reacts to produce hydrogen gas and a corresponding salt. This reaction is similar to the fizz you see when vinegar meets baking soda, though it involves different substances.
Equation: Al-Cu-Mn-Mg alloy+HCl→H₂ (gas)+Salt
Duralumin conducts electricity fairly well, but not as efficiently as pure aluminum. This slight decrease in conductivity is due to the additional metals mixed with aluminum, which slightly impede the flow of electricity.
The process of heat treatment, particularly aging, makes duralumin stronger. This process isn’t a chemical reaction but rather a change in the material’s internal structure that enhances its physical properties.
Anodizing thickens duralumin’s natural oxide layer, enhancing its resistance to corrosion and wear. This process can be likened to upgrading its natural protective shield, making it tougher against environmental challenges.
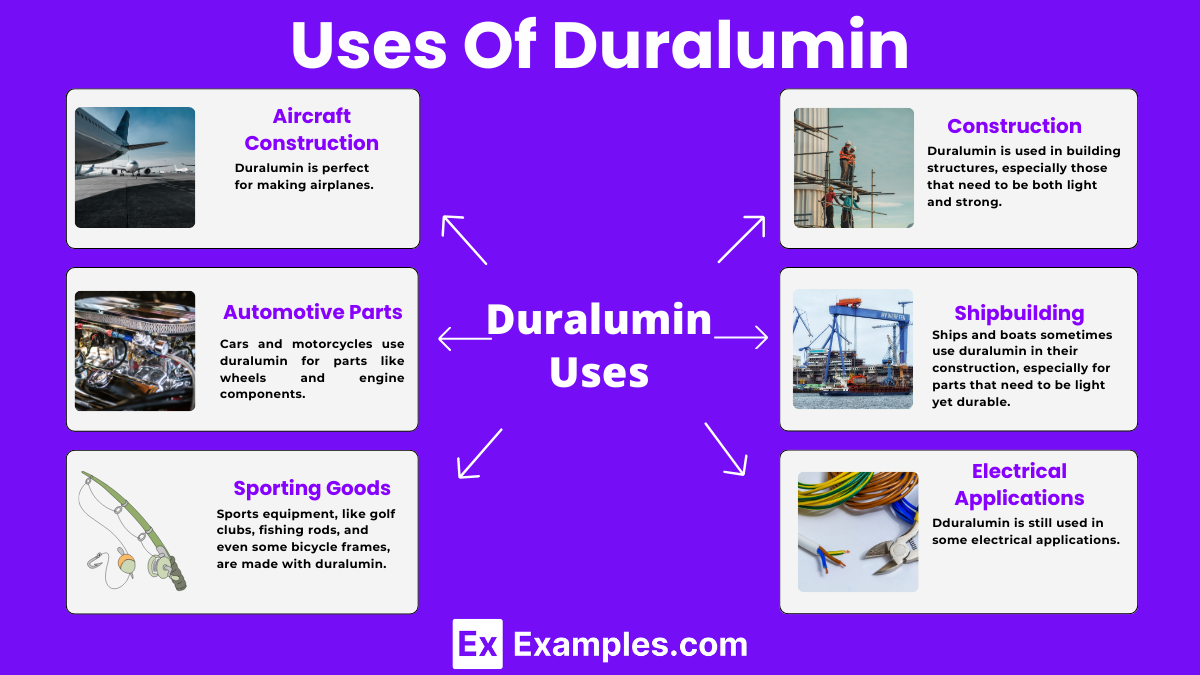
Duralumin is perfect for making airplanes. Its lightness helps the plane fly high while its strength ensures the plane is safe and sturdy, even at high speeds or in bad weather.
Cars and motorcycles use duralumin for parts like wheels and engine components. It helps make the vehicles lighter, which can lead to better fuel efficiency and speed, and it’s tough enough to handle the wear and tear of the road.
Sports equipment, like golf clubs, fishing rods, and even some bicycle frames, are made with duralumin. It makes the gear lightweight for better performance and strong enough to withstand lots of use.
Duralumin is used in building structures, especially those that need to be both light and strong. It’s found in bridges, roofs, and frames for buildings, offering durability and resistance to weather conditions.
Ships and boats sometimes use duralumin in their construction, especially for parts that need to be light yet durable. It helps in improving the speed and efficiency of maritime vehicles while ensuring they are robust against the harsh conditions of the sea.
Though not as conductive as pure aluminum, duralumin is still used in some electrical applications where strength and a bit of conductivity are needed, like in certain types of electrical cables and connectors.
One of the biggest advantages of duralumin is its exceptional strength without being heavy. This makes it perfect for aircraft and automotive parts, where reducing weight is crucial for efficiency and performance.
Duralumin is resistant to rust and corrosion, thanks to the protective layer of aluminum oxide that forms on its surface. This makes it ideal for use in environments where moisture and corrosion are concerns, such as in marine applications and outdoor equipment.
The alloy is known for its durability and ability to withstand stress and strain without breaking or deforming. This makes it a reliable material for construction and manufacturing, where long-lasting materials are needed.
Duralumin can be easily shaped, welded, and machined into complex forms, allowing for flexibility in design and manufacturing. This property is particularly valuable in the aerospace and automotive industries, where precision and custom shapes are required.
While it doesn’t conduct electricity as well as pure aluminum, duralumin still offers sufficient conductivity for certain electrical applications. This makes it useful for some types of electrical cables and components.
Compared to other high-strength materials, duralumin provides a cost-effective solution. Its combination of low cost and high performance makes it an attractive choice for many industries, balancing quality with affordability.
Duralumin, primarily made of aluminum, does not rust like iron. It forms a protective oxide layer, providing good resistance to corrosion.
While not as strong as steel, duralumin offers a superior strength-to-weight ratio, making it ideal for applications where lightweight materials are preferred.
Duralumin is also known as duraluminum, emphasizing its base of aluminum combined with copper to enhance its strength and durability.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary base metal in Duralumin?
Copper
Aluminum
Zinc
Nickel
Which metal is not typically added to Duralumin?
Copper
Magnesium
Iron
Silicon
Duralumin is known for its:
High density
High corrosion resistance
High strength-to-weight ratio
High thermal conductivity
After heat treatment, Duralumin needs to be:
Quenched in water
Left at room temperature
Aged naturally
Aged artificially
Duralumin was first developed in:
Germany
United States
France
Russia
Which industry is Duralumin most commonly used in?
Culinary
Aerospace
Jewelry
Construction
The typical alloying element of Duralumin that enhances its strength is:
Lead
Tin
Copper
Zinc
Which property of Duralumin decreases as a result of its aging process?
Elasticity
Ductility
Hardness
Malleability
What is a disadvantage of using Duralumin?
High cost
Prone to corrosion
Easily weldable
Non-recyclable
Duralumin’s comparison with pure aluminum shows that it is:
More ductile
Less reflective
Heavier
Stronger
Before you leave, take our quick quiz to enhance your learning!

