What is the atomic number of Dysprosium?
64
65
66
67
Discover the wonders of Dysprosium, a lesser-known yet incredibly valuable element in the periodic table. This complete guide illuminates Dysprosium’s unique properties, its pivotal role in modern technology, and its various applications. Through detailed examples, we’ll explore how Dysprosium enhances high-tech devices, contributes to green energy solutions, and stands out in scientific research. Dive into the world of Dysprosium to uncover its significance, uses, and the innovative compounds it forms, enriching our technological landscape.
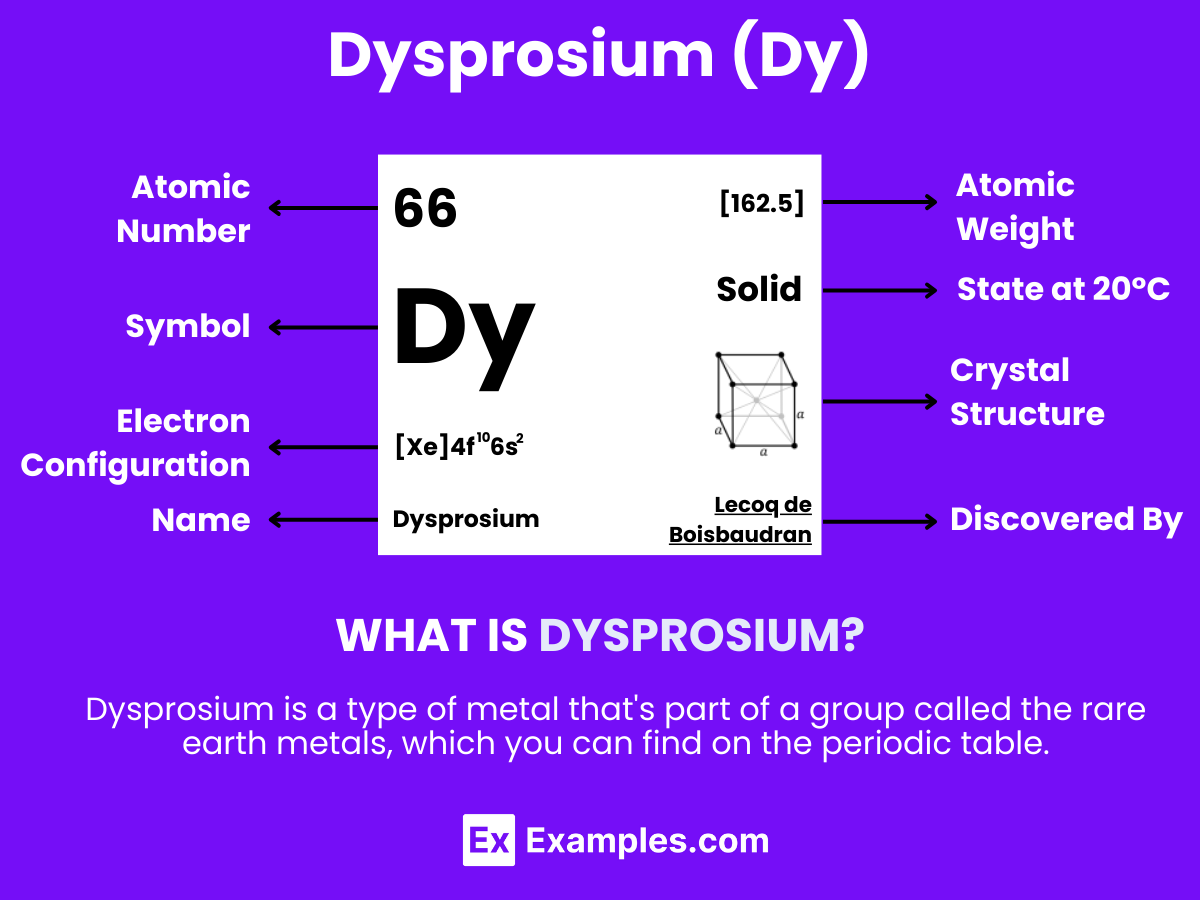
Dysprosium is a chemical element with the symbol Dy and atomic number 66. It is one of the lanthanides, a series of rare earth elements, and is known for its metallic and bright silver luster. Dysprosium has unique properties that make it highly valuable in various high-tech applications. Despite being called a “rare” earth element, dysprosium is relatively abundant in the Earth’s crust, comparable to some common metals like lead, but it is difficult to extract in pure form due to its presence in mixed rare earth minerals.
| Lanthanum | Gadolinium |
| Cerium | Terbium |
| Praseodymium | Lutetium |
| Neodymium | Holmium |
| Promethium | Erbium |
| Samarium | Thulium |
| Europium | Ytterbium |
| Physical Property | Value |
|---|---|
| Appearance | Silvery white, metallic |
| Melting Point | 1,412 °C (2,574 °F) |
| Boiling Point | 2,567 °C (4,653 °F) |
| Density | 8.540 g/cm³ at 20 °C |
| Phase at Room Temperature | Solid |
| Thermal Conductivity | 10.7 W/(m·K) |
| Electrical Resistivity | 926 nΩ·m at 20 °C |
| Magnetic Ordering | Paramagnetic |
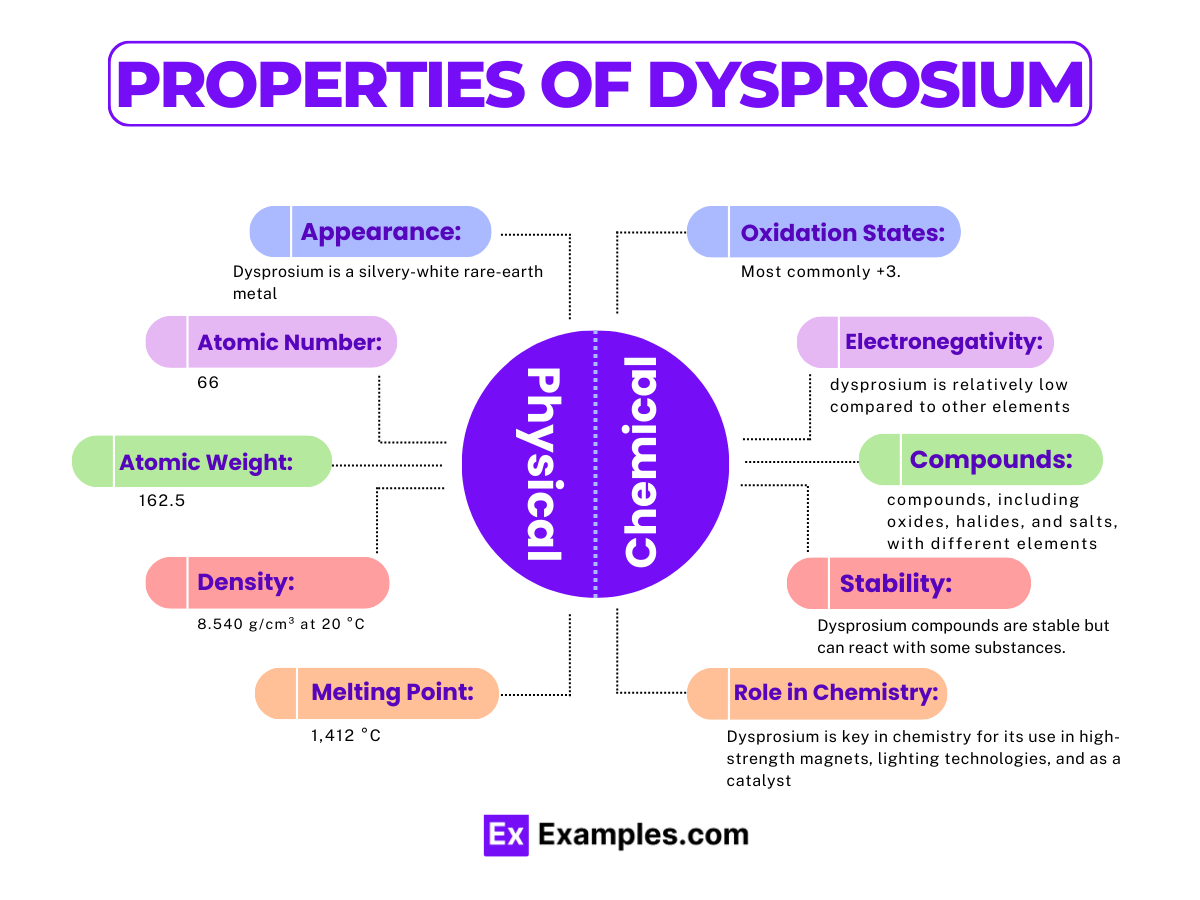
Dysprosium (Dy) is a rare earth element known for its large magnetic susceptibility. It belongs to the lanthanide series in the periodic table and exhibits interesting chemical properties due to its electron configuration. Here, we delve into the chemical properties of dysprosium, emphasizing its reactions, common oxidation states, and examples of its compounds.
Oxidation States: The most common oxidation state of dysprosium is +3 Dy³⁺. This oxidation state is prevalent in almost all dysprosium compounds, reflecting its stable electron configuration [Xe] 4f¹⁰ 6s².
Reactivity Dysprosium is relatively stable in dry air but oxidizes in moist air, forming dysprosium(III) oxide:
3Dy + O₂ → 2Dy₂O₃
This reaction demonstrates dysprosium’s reactivity towards oxygen, leading to the formation of a white oxide layer on the metal’s surface.
Reaction with Water Dysprosium reacts with water, albeit slowly, to form dysprosium hydroxide and hydrogen gas:
2 Dy+6 H2O→2 Dy(OH)₃+3 H₂
| Thermodynamic Property | Value |
|---|---|
| Melting Point | 1,412 °C |
| Boiling Point | 2,567 °C |
| Standard Molar Entropy (S°) | 192.0 J/(mol·K) |
| Standard Enthalpy of Formation (ΔHf°) | -185 kJ/mol (for Dy2O3) |
| Material Property | Value |
|---|---|
| Crystal Structure | Hexagonal close-packed (hcp) |
| Hardness | Relatively soft, malleable |
| Modulus of Elasticity | 61.4 GPa |
| Thermal Expansion | 9.9 µm/(m·K) at 25 °C |
| Electromagnetic Property | Value |
|---|---|
| Electrical Resistivity | 926 nΩ·m at 20 °C |
| Magnetic Ordering | Paramagnetic at 300 K |
| Curie Temperature | Approximately 88 K |
| Nuclear Property | Value |
|---|---|
| Natural Isotopes | Dy-156, Dy-158, Dy-160, Dy-161, Dy-162, Dy-163, Dy-164 |
| Most Stable Isotope | Dy-164 with a half-life of over 1.8×10141.8×1014 years |
| Neutron Cross Section | 994 barns (for Dy-164) |
| Isotopic Abundance | Dy-164: 28.18%, Dy-162: 25.51%, etc. |
The preparation of dysprosium typically involves the extraction and purification processes from minerals like monazite and bastnäsite, which contain dysprosium in the form of oxides. The process begins with the crushing of these minerals, followed by acid or alkali treatments to produce a mixture of rare earth chlorides. Dysprosium is then separated from other rare earths through solvent extraction or ion exchange techniques. Finally, the dysprosium chloride is reduced with metallic calcium in a high-temperature vacuum environment to produce pure dysprosium metal: DyCl₃ + 3Ca → Dy + 3CaCl₂
The production of dysprosium involves complex processes, primarily starting from the mining of rare earth-containing minerals like monazite and bastnäsite. The extraction process includes crushing the ore, acid leaching to obtain mixed rare earth chlorides or oxides, and then separating dysprosium from other rare earths using solvent extraction or ion-exchange methods. The final step involves reducing dysprosium fluoride with calcium metal in an inert atmosphere to produce pure dysprosium metal.

Discover the wonders of Dysprosium, a lesser-known yet incredibly valuable element in the periodic table. This complete guide illuminates Dysprosium’s unique properties, its pivotal role in modern technology, and its various applications. Through detailed examples, we’ll explore how Dysprosium enhances high-tech devices, contributes to green energy solutions, and stands out in scientific research. Dive into the world of Dysprosium to uncover its significance, uses, and the innovative compounds it forms, enriching our technological landscape.
Dysprosium is a chemical element with the symbol Dy and atomic number 66. It is one of the lanthanides, a series of rare earth elements, and is known for its metallic and bright silver luster. Dysprosium has unique properties that make it highly valuable in various high-tech applications. Despite being called a “rare” earth element, dysprosium is relatively abundant in the Earth’s crust, comparable to some common metals like lead, but it is difficult to extract in pure form due to its presence in mixed rare earth minerals.
Formula: Dy
Composition: Dysprosium is composed entirely of dysprosium atoms, categorizing it as an elemental substance.
Bond Type: As an element, dysprosium in its pure form does not engage in bonding. However, it is capable of forming diverse chemical bonds, such as ionic and covalent, with other elements. This characteristic enables dysprosium to participate in the formation of a variety of compounds.
Molecular Structure: Dysprosium, in its elemental state, does not have a molecular structure. It possesses a metallic structure, typically manifesting in a hexagonal close-packed crystalline form. This structure reflects dysprosium’s nature as a dense, silvery metal, which may exhibit a slight metallic luster.
Electron Sharing: Dysprosium can share electrons to establish covalent bonds or donate electrons to create ionic bonds. In its compounds, dysprosium often appears in a +3 oxidation state (Dy³⁺), showcasing its ability to form a wide range of chemical species.
Significance: Dysprosium plays a significant role across various fields, notably in the manufacturing of neodymium magnets, which are crucial for modern technologies such as electric motors and wind turbine generators. Its high thermal neutron absorption cross-section makes it useful in nuclear reactors as a control material.
Role in Chemistry: The chemical properties of dysprosium, a member of the lanthanide series, attract attention in materials science and technological applications, emphasizing the unique characteristics of rare earth elements. Its compounds are utilized in making laser materials and phosphors for lighting and displays. Dysprosium’s behavior in compounds and its interactions with other elements are crucial for advancing materials science and developing new technologies for magnetic, luminescent, and high-strength materials.

Elemental Overview
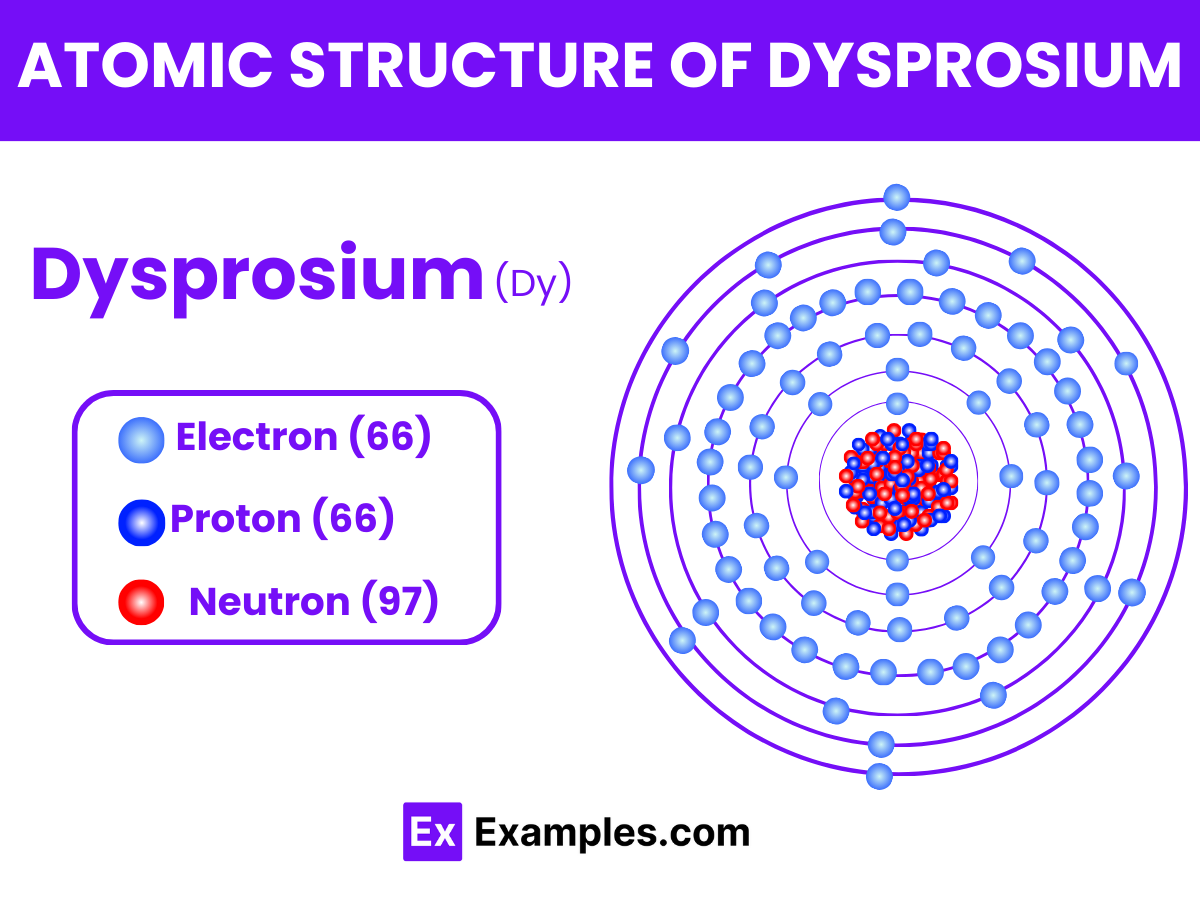
Dysprosium (Dy) is a chemical element with the atomic number 66.
It belongs to the lanthanide series in the periodic table.
Atomic Number and Mass
Atomic Number: 66
Atomic Mass: Approximately 162.5 atomic mass units (amu).
Electron Configuration
Dysprosium’s electron configuration is Xe 4f¹⁰6s²
It has a total of 66 electrons distributed among its shells.
Orbital Diagram
Dysprosium has a full 4f shell and Dy 4f¹⁰6s² electrons in its outermost shell.
Atomic Structure
Dysprosium consists of 66 protons in its nucleus.
It typically contains 97 neutrons, resulting in an atomic mass of approximately 162.5 amu.
The nucleus of dysprosium is surrounded by 66 electrons.

Physical Property | Value |
|---|---|
Appearance | Silvery white, metallic |
Melting Point | 1,412 °C (2,574 °F) |
Boiling Point | 2,567 °C (4,653 °F) |
Density | 8.540 g/cm³ at 20 °C |
Phase at Room Temperature | Solid |
Thermal Conductivity | 10.7 W/(m·K) |
Electrical Resistivity | 926 nΩ·m at 20 °C |
Magnetic Ordering | Paramagnetic |
Dysprosium (Dy) is a rare earth element known for its large magnetic susceptibility. It belongs to the lanthanide series in the periodic table and exhibits interesting chemical properties due to its electron configuration. Here, we delve into the chemical properties of dysprosium, emphasizing its reactions, common oxidation states, and examples of its compounds.
Oxidation States: The most common oxidation state of dysprosium is +3 Dy³⁺. This oxidation state is prevalent in almost all dysprosium compounds, reflecting its stable electron configuration [Xe] 4f¹⁰ 6s².
Reactivity Dysprosium is relatively stable in dry air but oxidizes in moist air, forming dysprosium(III) oxide:
3Dy + O₂ → 2Dy₂O₃
This reaction demonstrates dysprosium’s reactivity towards oxygen, leading to the formation of a white oxide layer on the metal’s surface.
Reaction with Water Dysprosium reacts with water, albeit slowly, to form dysprosium hydroxide and hydrogen gas:
2 Dy+6 H2O→2 Dy(OH)₃+3 H₂
Thermodynamic Property | Value |
|---|---|
Melting Point | 1,412 °C |
Boiling Point | 2,567 °C |
Standard Molar Entropy (S°) | 192.0 J/(mol·K) |
Standard Enthalpy of Formation (ΔHf°) | -185 kJ/mol (for Dy2O3) |
Material Property | Value |
|---|---|
Crystal Structure | Hexagonal close-packed (hcp) |
Hardness | Relatively soft, malleable |
Modulus of Elasticity | 61.4 GPa |
Thermal Expansion | 9.9 µm/(m·K) at 25 °C |
Electromagnetic Property | Value |
|---|---|
Electrical Resistivity | 926 nΩ·m at 20 °C |
Magnetic Ordering | Paramagnetic at 300 K |
Curie Temperature | Approximately 88 K |
Nuclear Property | Value |
|---|---|
Natural Isotopes | Dy-156, Dy-158, Dy-160, Dy-161, Dy-162, Dy-163, Dy-164 |
Most Stable Isotope | Dy-164 with a half-life of over 1.8×10141.8×1014 years |
Neutron Cross Section | 994 barns (for Dy-164) |
Isotopic Abundance | Dy-164: 28.18%, Dy-162: 25.51%, etc. |
The preparation of dysprosium typically involves the extraction and purification processes from minerals like monazite and bastnäsite, which contain dysprosium in the form of oxides. The process begins with the crushing of these minerals, followed by acid or alkali treatments to produce a mixture of rare earth chlorides. Dysprosium is then separated from other rare earths through solvent extraction or ion exchange techniques. Finally, the dysprosium chloride is reduced with metallic calcium in a high-temperature vacuum environment to produce pure dysprosium metal: DyCl₃ + 3Ca → Dy + 3CaCl₂

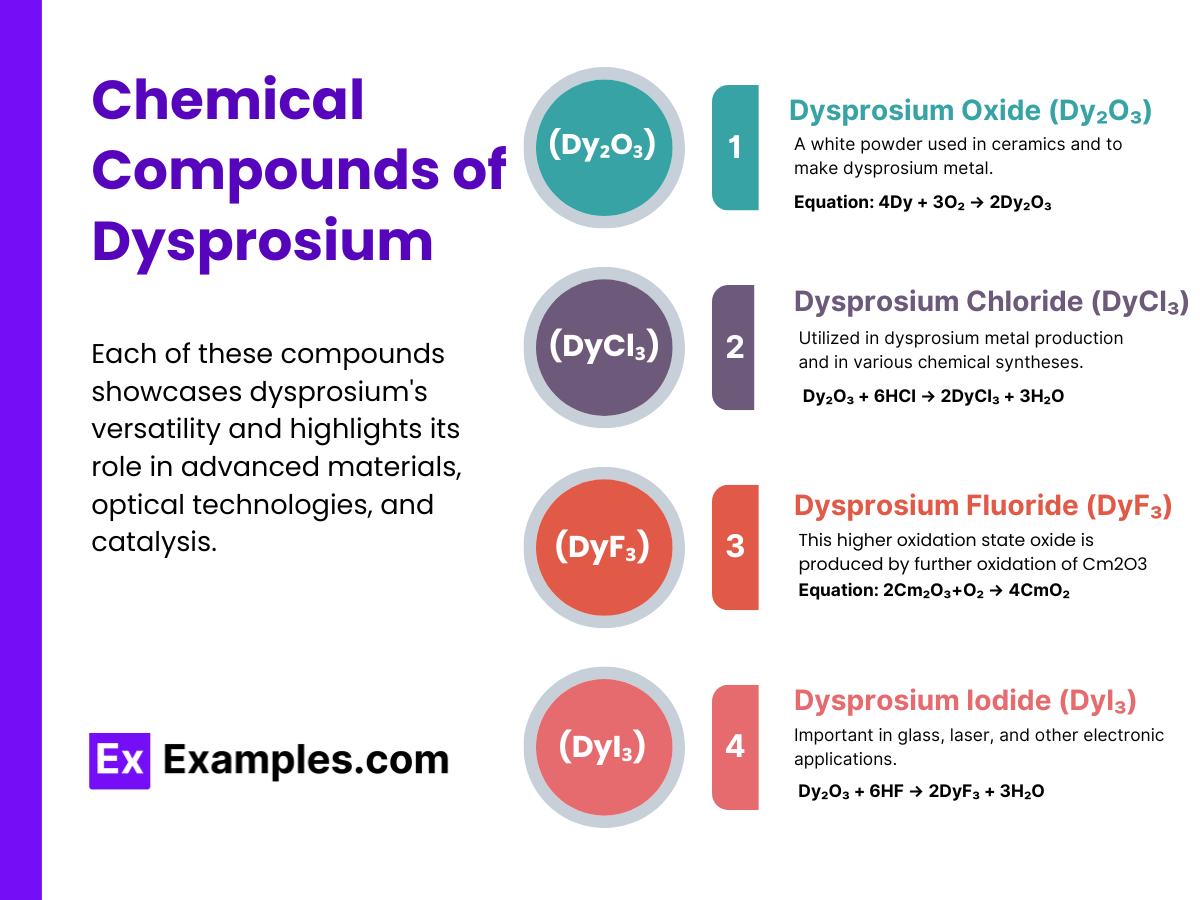
Dysprosium Oxide (Dy₂O₃):A white powder used in ceramics and to make dysprosium metal. It’s prepared by heating dysprosium in air:
4Dy + 3O₂ → 2Dy₂O₃
Dysprosium Chloride (DyCl₃):Utilized in dysprosium metal production and in various chemical syntheses. It can be produced by reacting dysprosium oxide with hydrochloric acid:
Dy₂O₃ + 6HCl → 2DyCl₃ + 3H₂O
Dysprosium Fluoride (DyF₃):Important in glass, laser, and other electronic applications. It is synthesized by reacting dysprosium oxide with hydrofluoric acid:
Dy₂O₃ + 6HF → 2DyF₃ + 3H₂O
Dysprosium Iodide (DyI₃):Used in specialty lighting and electronics, it can be prepared by reacting dysprosium metal with iodine:
2Dy + 3I₂ → 2DyI₃
Dysprosium Nitrate (Dy(NO₃): A soluble compound used in certain catalysis and material science research. It’s derived from the reaction of dysprosium oxide with nitric acid:
Dy₂O₃ + 6HNO₃ → 2Dy(NO₃)₃ + 3H₂O
Dysprosium Sulfide (Dy2S₃):It finds applications in electronic devices. Prepared by reacting dysprosium with sulfur:
2Dy + 3S → Dy₂S₃

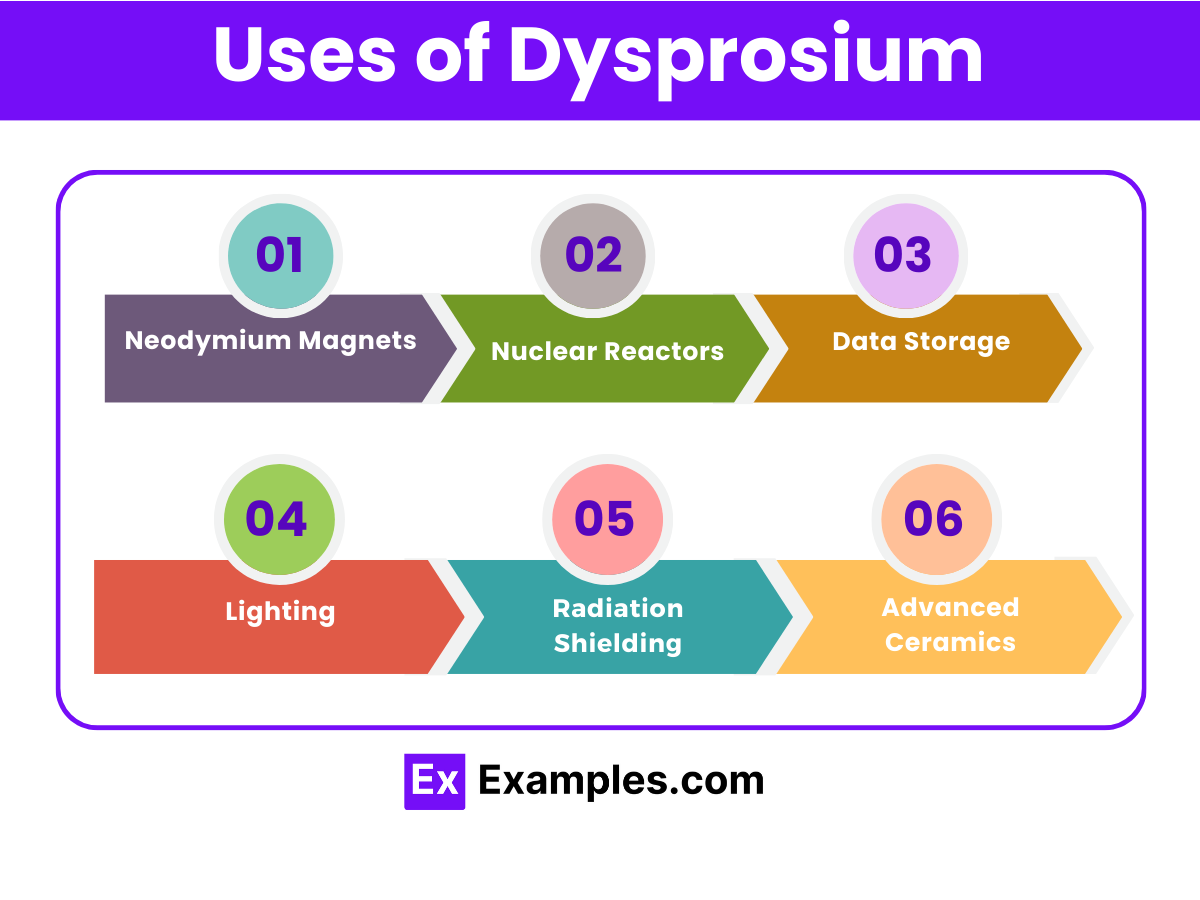
Neodymium Magnets: Dysprosium is added to neodymium magnets to improve their heat resistance, making them ideal for use in high-performance motors and electric vehicles.
Nuclear Reactors: It serves as a neutron absorber in nuclear reactors, helping to control the nuclear reaction and maintain safety.
Data Storage: Dysprosium is used in data storage applications, including hard disks, due to its magnetic properties, enhancing storage capacity and efficiency.
Lighting: It’s utilized in making lighting sources, such as halide lamps, where it contributes to producing a bright, white light.
Radiation Shielding: Dysprosium compounds can be used in radiation shielding materials to absorb neutrons and protect against radiation damage.
Advanced Ceramics: Incorporated into various ceramics and glass to improve their thermal and mechanical properties, dysprosium is crucial for manufacturing advanced ceramics used in electronics and high-tech applications.
The production of dysprosium involves complex processes, primarily starting from the mining of rare earth-containing minerals like monazite and bastnäsite. The extraction process includes crushing the ore, acid leaching to obtain mixed rare earth chlorides or oxides, and then separating dysprosium from other rare earths using solvent extraction or ion-exchange methods. The final step involves reducing dysprosium fluoride with calcium metal in an inert atmosphere to produce pure dysprosium metal.
Neodymium-Iron-Boron (NdFeB) Magnets: Dysprosium is added to NdFeB magnets to enhance their thermal stability, crucial for high-performance and high-temperature applications such as in electric vehicles and wind turbines.
Nuclear Reactors: Its ability to absorb neutrons without swelling or contracting makes dysprosium a valuable material in nuclear reactor control rods, helping in the regulation of the reactor’s fission rate.
Data Storage: Dysprosium’s magnetic properties are utilized in the manufacturing of hard disks and data storage devices, providing higher data storage capacity and stability.
Lasers: Certain dysprosium compounds are used in making laser materials for cutting, welding, and other industrial applications.
Lighting: Dysprosium iodide is used in high-intensity metal halide lamps, where it contributes to the production of bright, white light with high color rendering.
Dysprosium stands as a cornerstone in the advancement of technology and industry. Its unique magnetic, nuclear, and luminescent properties underpin critical applications in magnets, nuclear reactors, and lighting. As we explore its potential, dysprosium’s role in green technology and electronics continues to expand, underscoring the importance of sustainable extraction and utilization of this invaluable rare earth element.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Dysprosium?
64
65
66
67
Dysprosium belongs to which group of elements?
Alkaline earth metals
Transition metals
Lanthanides
Halogens
What is the primary use of Dysprosium in modern technology?
Construction materials
Magnets in electric motors
Fertilizers
Jewelry making
What is the symbol of Dysprosium in the periodic table?
Dy
Dp
Ds
Do
In which type of reactors is Dysprosium used to control nuclear fission?
Fusion reactors
Breeder reactors
Fast-neutron reactors
Nuclear fission reactors
What is the primary oxidation state of Dysprosium?
+1
+2
+3
+4
What is the natural state of Dysprosium at room temperature?
Gas
Liquid
Solid
Plasma
Which property of Dysprosium makes it useful in nuclear control rods?
High electrical conductivity
High neutron absorption
Low melting point
Low thermal conductivity
Dysprosium is often alloyed with which other element to improve its magnetic properties?
Iron
Copper
Zinc
Gold
In which year was Dysprosium discovered?
1867
1886
1899
1903
Before you leave, take our quick quiz to enhance your learning!

