What is the atomic number of einsteinium?
97
98
99
100
Embark on an exploratory journey into the atomic world of Einsteinium, a synthetic element shrouded in the mysteries of nuclear science. This complete guide unveils the enigmatic properties, production methods, and the pivotal role of Einsteinium in advancing scientific knowledge. With examples ranging from its discovery in the aftermath of nuclear tests to its contributions to research in chemistry and physics, we delve into how Einsteinium serves as a key to unlocking profound insights into the structure of the atomic nucleus and the potential for new chemical innovations. Discover the intriguing applications and challenges of harnessing Einsteinium, an element that encapsulates the relentless pursuit of scientific discovery.
Einsteinium has no stable isotopes, with Einsteinium-252 being one of its most studied isotopes due to its relatively longer half-life of about 471.7 days. The element exhibits chemical properties typical of the actinide series and is primarily used for research purposes. Scientists study einsteinium to learn more about its properties and to synthesize new elements and compounds in the lab. Due to its scarcity and radioactivity, practical applications of einsteinium outside of scientific research are limited
| Actinium | Berkelium |
| Thorium | Californium |
| Protactinium | Fermium |
| Uranium | Curium |
| Neptunium | Mendelevium |
| Plutonium | Nobelium |
| Americium | Lawrencium |
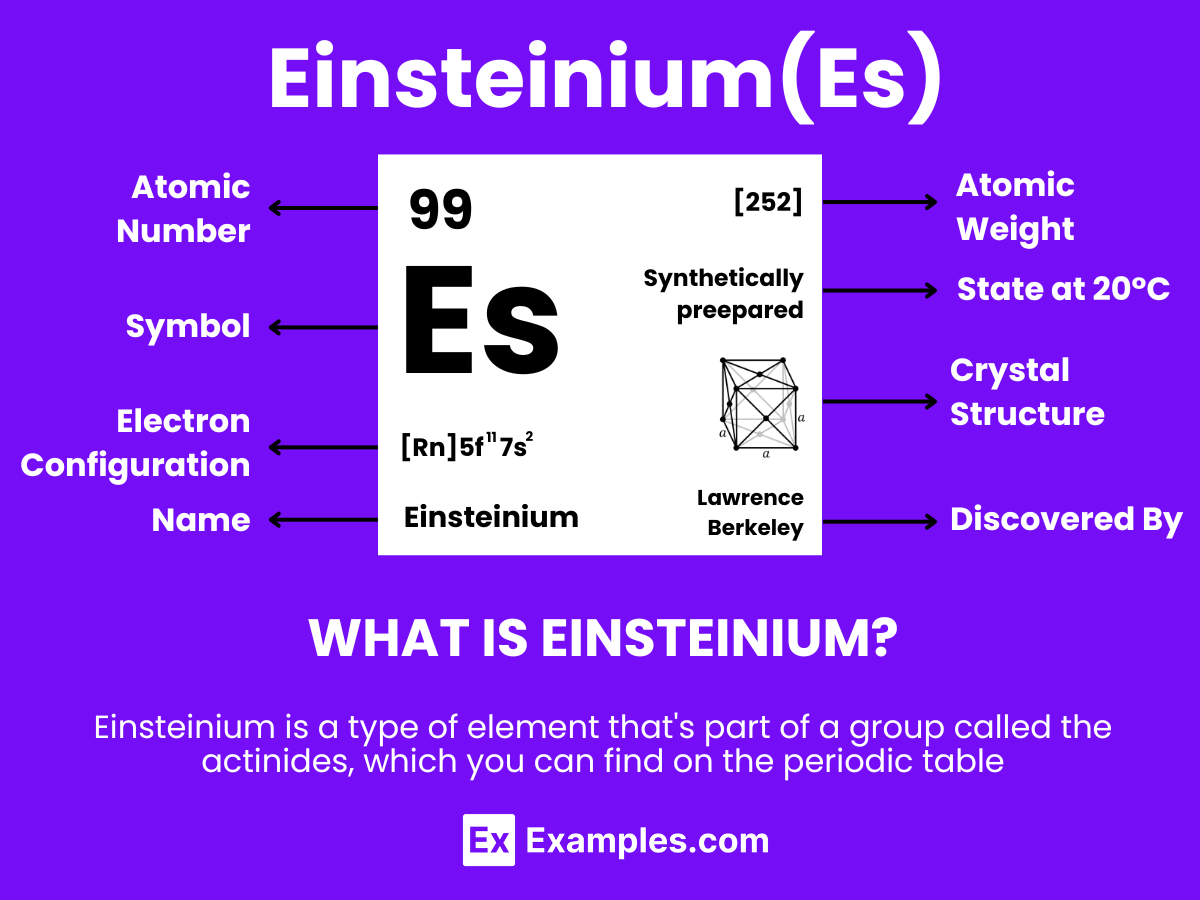
Einsteinium, with the symbol Es and atomic number 99, is a synthetic element that belongs to the actinide series of the periodic table. Known for its radioactivity and synthetic origin, Einsteinium has a complex atomic structure that contributes to its unique chemical and physical properties. Here’s a breakdown of the atomic structure of Einsteinium:
| Property | Value |
|---|---|
| Appearance | Silvery, lustrous, almost white metal |
| Phase at Room Temperature | Solid |
| Density | Approx. 8.84 g/cm³ (estimated) |
| Melting Point | 860°C (estimated) |
| Boiling Point | Estimated to be around 996°C |
| Atomic Mass | 252 u (most stable isotope, Einsteinium-252) |
| Crystal Structure | Face-centered cubic (fcc) |
| State of Matter | Radioactive metal |
Einsteinium, with the symbol Es and atomic number 99, is a synthetic, highly radioactive element belonging to the actinide series. Its chemical properties share similarities with other actinides, exhibiting complex behavior due to its radioactivity and position in the periodic table. Here we explore the detailed chemical properties of Einsteinium, focusing on its oxidation states, reactions, and notable compounds.
| Property | Value |
|---|---|
| Melting Point | Approximately 860°C |
| Boiling Point | Estimated to be around 996°C |
| Heat of Fusion | Data not available |
| Heat of Vaporization | Data not available |
| Specific Heat Capacity | Data not available |
| Property | Value |
|---|---|
| State at Room Temperature | Solid |
| Density | Estimated to be around 8.84 g/cm³ |
| Appearance | Silver-colored, radioactive metal |
| Crystal Structure | Face-centered cubic (estimated) |
| Property | Value |
|---|---|
| Electrical Resistivity | High (specific value not available) |
| Magnetic Ordering | Paramagnetic |
| Thermal Conductivity | Data not available |
| Property | Value |
|---|---|
| Most Stable Isotope | Einsteinium-252 (half-life of 471.7 days) |
| Primary Decay Modes | Alpha decay |
| Neutron Cross Section | Data not available |
| Critical Mass | Not applicable due to production in microgram quantities |
The preparation of Einsteinium (Es) involves complex nuclear reactions, primarily occurring within high-flux nuclear reactors or during the detonation of nuclear weapons. Here’s a step-by-step overview of how Einsteinium is typically produced:
Description: A basic oxide, demonstrating Einsteinium’s ability to form compounds with oxygen. Equation: 2Es+23O₂→Es₂O₃
Description: Forms by reacting Einsteinium with chlorine, showing +3 oxidation state.
Equation: 3Es+23Cl₂→EsCl₃
Description: A compound indicating Einsteinium’s reactivity with halogens.
Equation: 3Es+23I₂→EsI₃
Description: Showcases Einsteinium’s ability to form stable halide compounds.
Equation: 3Es+23F₂→EsF₃
Description: Highlights Einsteinium’s consistent +3 oxidation state with halogens.
Equation: 3Es+23Br₂→EsBr₃
Description: Demonstrates Einsteinium’s reaction with water, forming hydroxides.
Equation: Es(OH)₃+3H+→Es₃++3H₂O
| Isotope | Half-Life | Decay Mode |
|---|---|---|
| Einsteinium-252 | 471.7 days | Alpha decay |
| Einsteinium-253 | 20.47 days | Beta decay |
| Einsteinium-254 | 275.7 days | Alpha decay |
| Einsteinium-255 | 39.8 days | Beta decay |
| Einsteinium-256 | 25.4 minutes | Beta decay |
| Einsteinium-257 | 7.7 days | Alpha decay |
The production of Einsteinium (Es) involves highly specialized nuclear reactions, primarily occurring within nuclear reactors or during high-energy nuclear tests. As a synthetic element, Einsteinium does not naturally exist on Earth and is produced through a series of neutron capture reactions and subsequent beta decays of heavier elements.
The applications of Einsteinium are predominantly in the field of scientific research, given its scarcity and high radioactivity. Here are the primary uses:
This article has systematically explored the intriguing world of Einsteinium, detailing its thermodynamic, material, electromagnetic, and nuclear properties through concise tables. Despite the challenges posed by its radioactivity and limited availability, the study of Einsteinium offers valuable insights into the actinide series and contributes significantly to scientific research, underscoring the element’s unique position in the periodic table and its potential for advancing our understanding of nuclear chemistry.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons (99)
Neutrons (153)
Protons (99)
What is the atomic number of einsteinium?
97
98
99
100
Einsteinium was named after which famous scientist?
Isaac Newton
Albert Einstein
Marie Curie
Niels Bohr
Einsteinium belongs to which group in the periodic table?
Lanthanides
Actinides
Transition metals
Halogens
What is the symbol for einsteinium?
Es
E
Ei
Et
Einsteinium was first discovered in the debris of which event?
Volcanic eruption
Nuclear explosion
Meteorite impact
Supernova
What is the most stable isotope of einsteinium?
Es-252
Es-253
Es-254
Es-255
Einsteinium is classified as what type of element?
Metal
Nonmetal
Metalloid
Noble gas
What color does einsteinium exhibit in its pure form?
Silver
Gold
Black
White
How was einsteinium first detected?
Through spectroscopy
By X-ray diffraction
Using a Geiger counter
By chemical reaction
Which of the following is a common use for einsteinium?
Medical imaging
Industrial catalyst
Scientific research
Jewelry makingAnswer: c. Scientific research
Explanation: Einsteinium is mainly used for scientific research due to its rarity and radioactivity.
Before you leave, take our quick quiz to enhance your learning!

