What is the atomic number of chlorine?
16
17
18
20
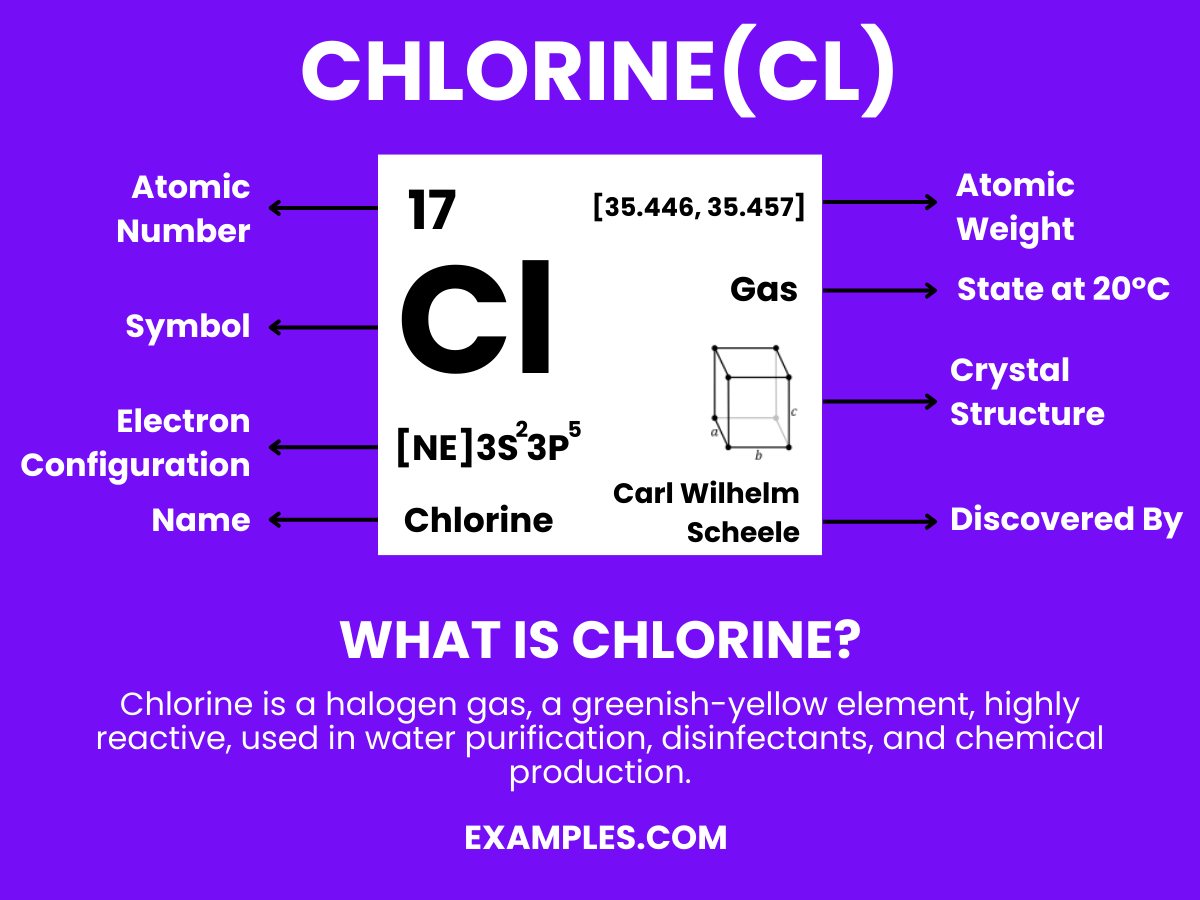
Chlorine, a highly reactive halogen, holds paramount importance in both organic and inorganic chemistry. This guide delves into chlorine’s multifaceted role, from water purification to the production of everyday household products. The element’s reactivity with hydrogen and other elements forms a myriad of compounds essential for various industrial applications. Discover the expansive world of chlorine, understand its interactions, especially with hydrogen, and explore its crucial role in maintaining public health and safety.
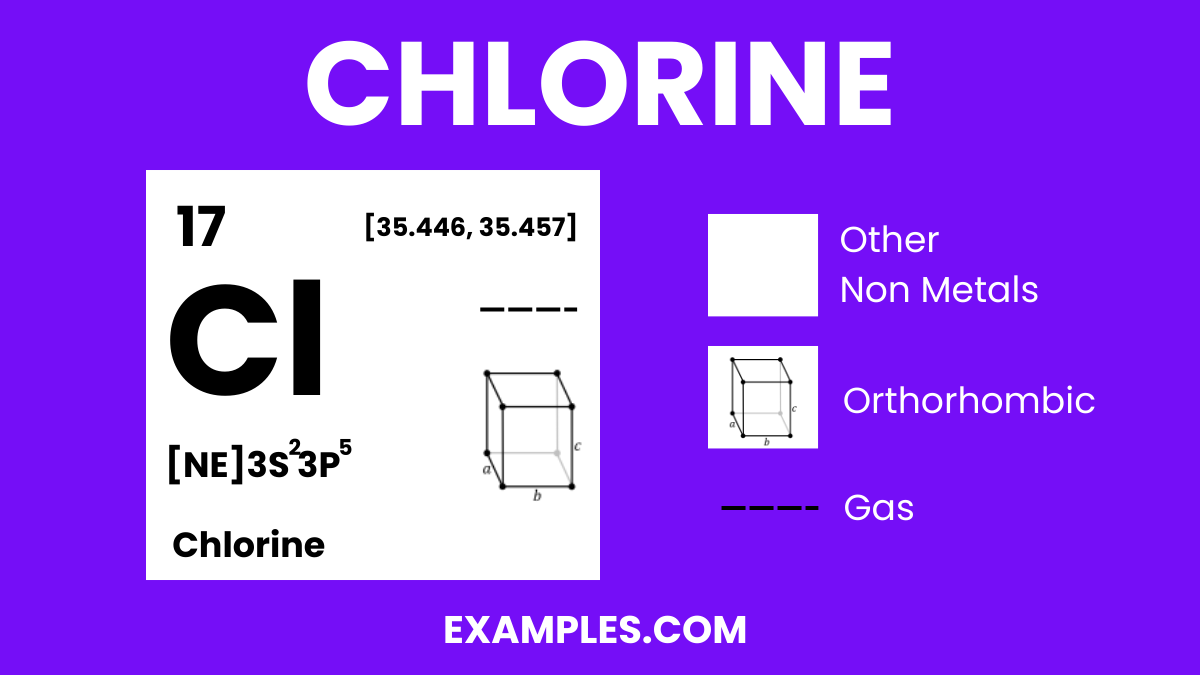
Chlorine is a greenish-yellow gas at room temperature, known for its powerful disinfectant properties and pungent odor. As a member of the halogen group, it is highly reactive, especially with hydrogen, forming hydrochloric acid, a substance fundamental to various industries. Chlorine is widely used in water treatment, bleaching, and the production of plastics and other chemicals. Its ability to eliminate bacteria and purify makes it essential in maintaining clean water supplies worldwide.
| Hydrogen | Phosphorus |
| Carbon | Sulfur |
| Nitrogen | Selenium |
| Oxygen | Bromine |
| Fluorine | Iodine |
Formula: Cl₂
Composition: Two chlorine atoms.
Bond Type: A single covalent bond connecting the atoms.
Molecular Structure: Diatomic molecule.
Electron Configuration: Seven valence electrons per atom, fourteen in total for Cl₂.
Significance: Essential for water purification, disinfectants, and bleach production.
Role in Chemistry: Widely used in the production of PVC, solvents, and many other organic compounds. Exists as a greenish-yellow gas at room temperature and has a strong, pungent odor.
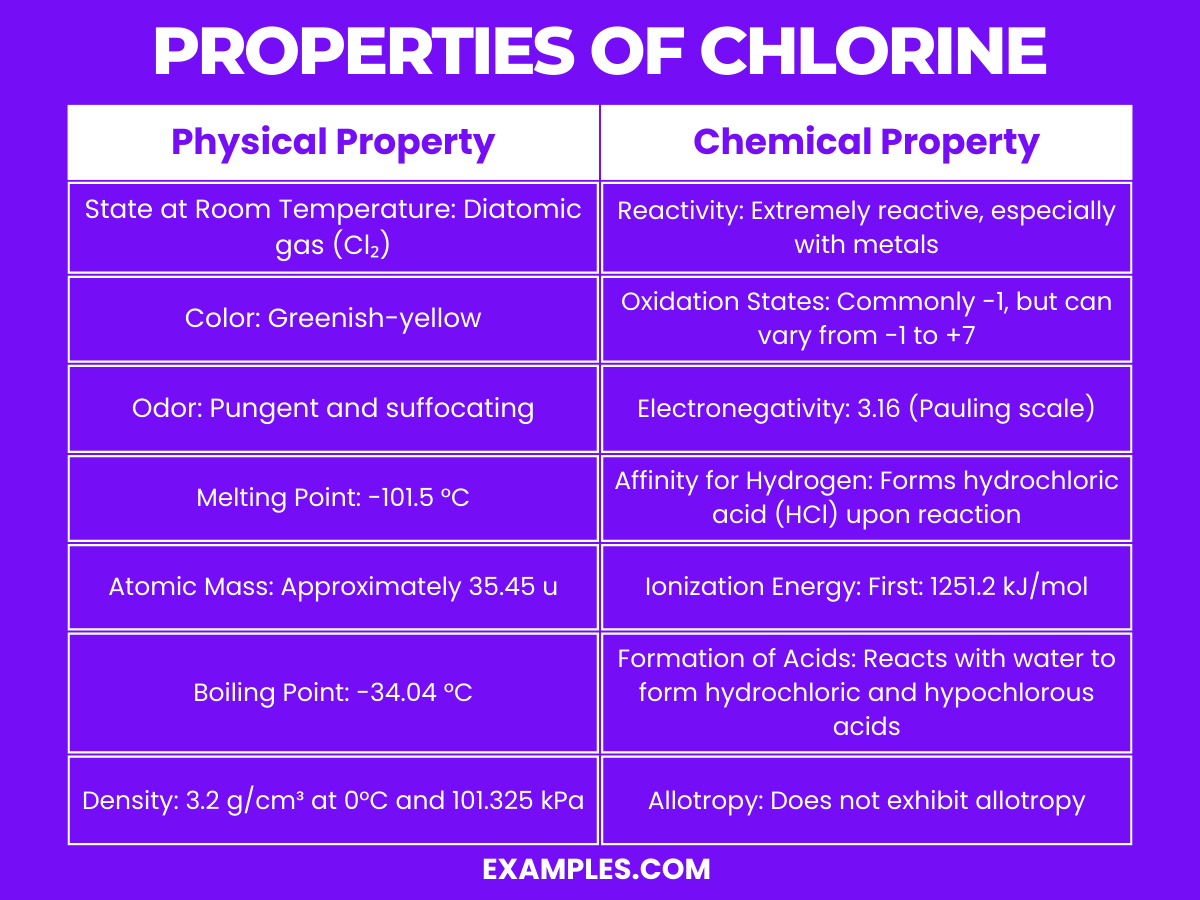
| Physical Property | Description |
|---|---|
| State at Room Temperature | Diatomic gas (Cl₂) |
| Color | Greenish-yellow |
| Odor | Pungent and suffocating, bleach-like |
| Melting Point | -101.5 °C |
| Boiling Point | -34.04 °C |
| Density | 3.2 g/cm³ at 0°C and 101.325 kPa (denser than air) |
| Solubility | Soluble in water, forming chloric acid and hydrochloric acid |
Chlorine is known for its high reactivity and potent oxidizing properties. Here are detailed explanations of its key chemical properties:
| Property | Value with Unit |
|---|---|
| Boiling Point | -34.04 °C |
| Melting Point | -101.5 °C |
| Critical Temperature | 144 °C |
| Critical Pressure | 7.99 MPa |
| Heat of Vaporization | 20.41 kJ/mol |
| Heat of Fusion | 6.40 kJ/mol |
| Specific Heat Capacity (at 25°C) | 0.479 J/g·K |
| Thermal Conductivity | 0.0089 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at 0°C and 1 atm) | 3.214 kg/m³ (Gas) |
| Viscosity (at -34°C) | 0.0135 mPa·s (Gas) |
| Solubility in Water (at 20°C) | 7.3 g/100 mL of water |
| Color | Greenish-yellow |
| Phase at Room Temperature | Gas |
| Property | Value with Unit |
|---|---|
| Electrical Conductivity | Non-conductive (Gas) |
| Electronegativity (Pauling scale) | 3.16 |
| Ionization Energy | 12.967 eV |
| Electron Affinity | 3.617 eV |
| Property | Value with Unit |
|---|---|
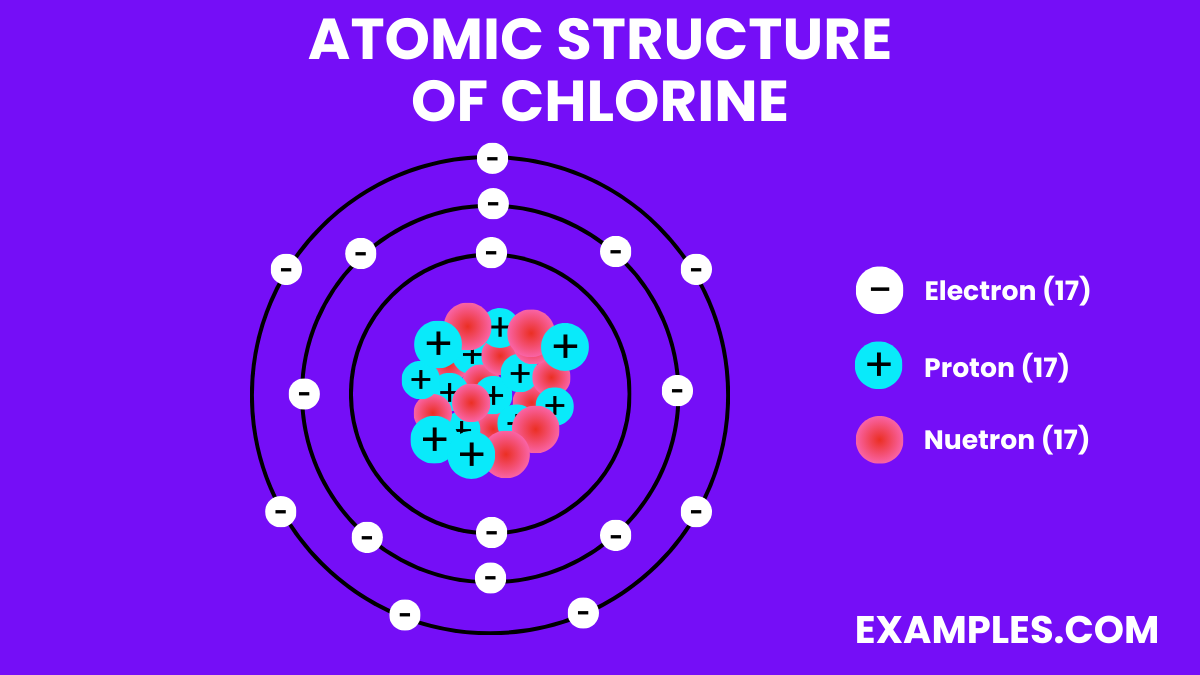
| Atomic Number | 17 |
| Atomic Mass | 35.453 amu (Average) |
| Isotopes | ^35Cl (75.77%), ^37Cl (24.23%) |
| Nuclear Spin (for ^35Cl) | 3/2 ℏ |
| Nuclear Spin (for ^37Cl) | 3/2 ℏ |
| Neutron Cross Section (for ^35Cl) | 43.6 barns |
| Neutron Cross Section (for ^37Cl) | 0.43 barns |
| Nuclear Magnetic Moment (for ^35Cl) | 0.821 µN |
| Nuclear Magnetic Moment (for ^37Cl) | 0.684 µN |
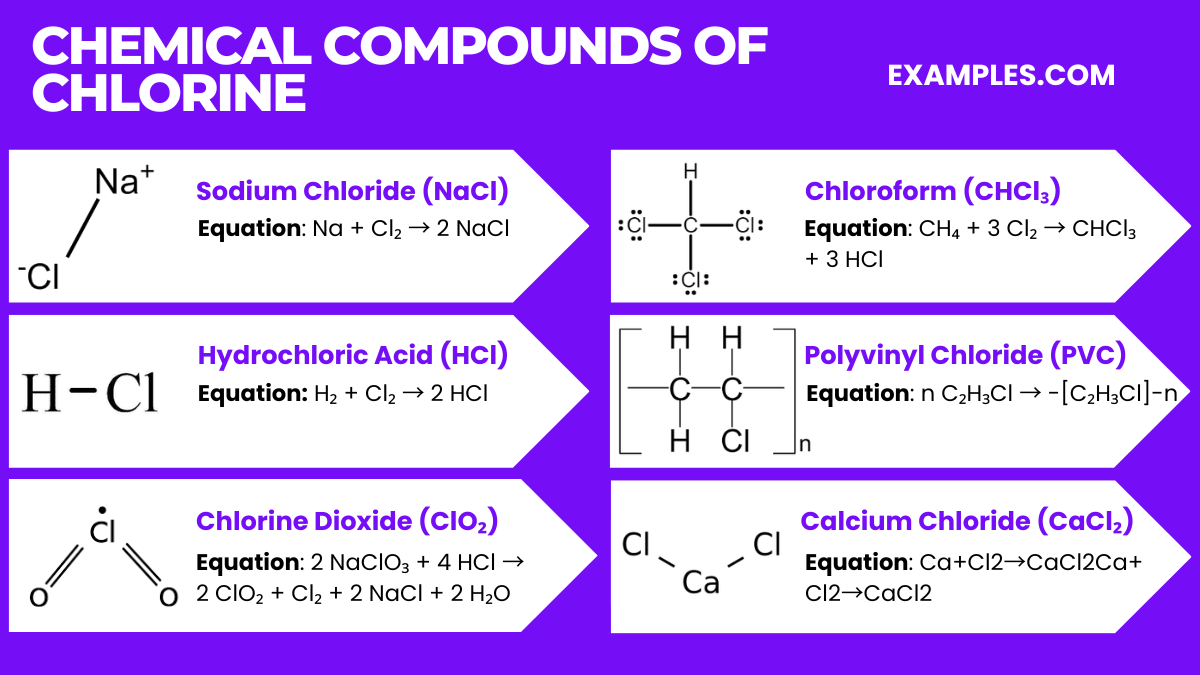
Chlorine, a highly reactive halogen, forms a wide array of significant chemical compounds, each with unique properties and applications. Some of the popular chlorine compounds are:
Chlorine has two stable isotopes, which are widely used in various applications. Below is a table detailing these isotopes:
| Isotope | Abundance (%) | Atomic Mass | Half-Life | Uses/Significance |
|---|---|---|---|---|
| Chlorine-35 | 75.77 | 34.969 amu | Stable | Used in the study of molecular structures via NMR spectroscopy. Predominant in nature, contributing to the average atomic mass of chlorine. |
| Chlorine-37 | 24.23 | 36.966 amu | Stable | Useful in scientific research and in nuclear reactions. It helps in understanding the chemical and physical properties of chlorine. |
These isotopes play crucial roles in both the natural environment and various scientific fields. Chlorine-35, being more abundant, is often the focus in studies related to chlorine’s behavior and reactions, while Chlorine-37’s unique properties make it valuable in specific research contexts.
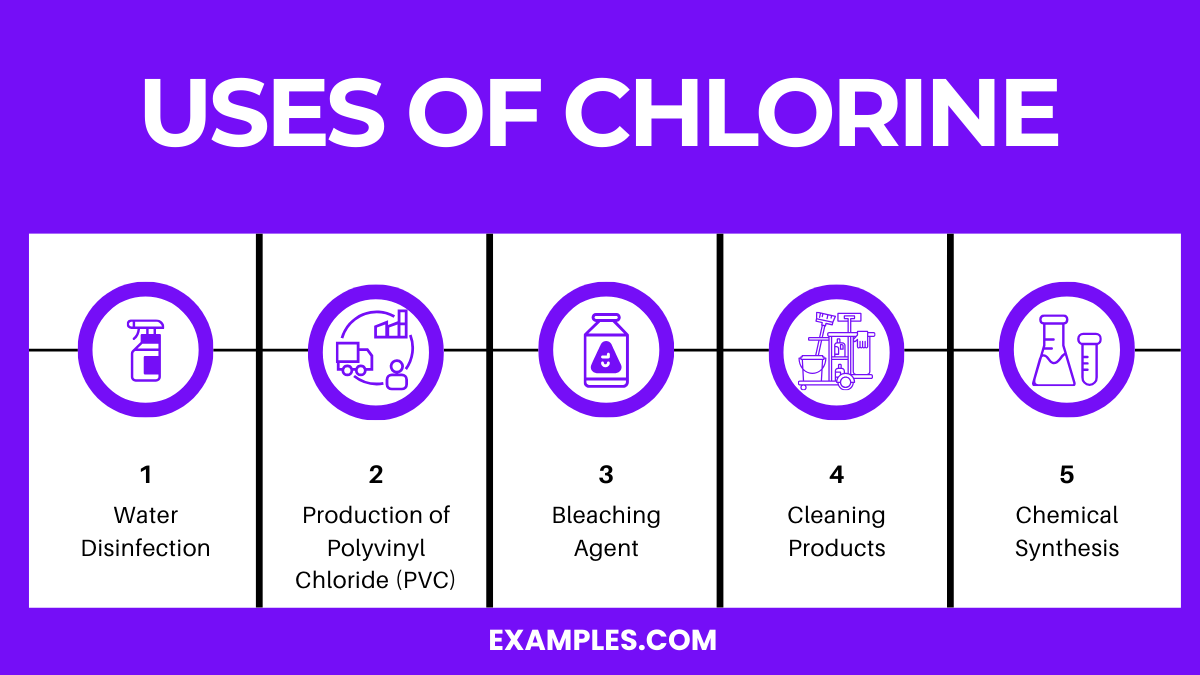
Chlorine is a chemical element with the symbol Cl and atomic number 17. It is a yellow-green gas under standard conditions, where it forms diatomic molecules. This element is an essential reagent in the chemical industry and has various applications, both industrial and household. Here are some of the most prominent uses of chlorine:
1. Water Disinfection: Chlorine is widely used for disinfecting drinking water and swimming pools. It kills bacteria, viruses, and other microbes that can cause disease. Chlorine is added to municipal water systems to make water safe for drinking. Its ability to eliminate pathogens has greatly reduced the incidence of waterborne diseases.
2. Production of Polyvinyl Chloride (PVC): PVC is a versatile plastic used in a wide range of products, including pipes, cables, frames, and medical devices. Chlorine is a key ingredient in producing PVC, making it an integral component in the plastics industry.
3. Bleaching Agent: Chlorine is a powerful bleaching agent used in the paper and textile industries. It is used to bleach wood pulp to produce white paper and to remove stains and colors from textiles.
4. Cleaning Products: Many household cleaning products and disinfectants contain chlorine or its compounds. These products are effective in killing germs, removing stains, and whitening surfaces.
5. Chemical Synthesis: Chlorine is involved in synthesizing various organic and inorganic chemicals, including solvents, dyes, and pharmaceuticals. It is used in producing hydrochloric acid, chloroform, and other important compounds.
6. Public Health and Sanitation: Chlorine compounds are used in sanitizing food processing equipment, controlling odor in waste treatment, and acting as an insecticide and a preservative. Its antimicrobial properties help maintain hygiene standards in various industries.
Chlorine is predominantly produced through a method known as the chlor-alkali process. This industrial process involves the electrolysis of sodium chloride (salt) solution, producing chlorine gas, sodium hydroxide, and hydrogen gas. Here are the key aspects of the commercial production of chlorine:
1. Chlor-Alkali Process: There are three main methods used in the chlor-alkali process: the mercury cell, the diaphragm cell, and the membrane cell. Each method uses a different technique to separate chlorine from the other products formed during electrolysis.
2. Raw Materials: The primary raw material for chlorine production is sodium chloride (NaCl), commonly known as table salt. This can be sourced from mined rock salt or evaporated from brine wells and seawater.
3. Energy Requirements: The chlor-alkali process is energy-intensive, requiring a significant amount of electricity to induce the necessary electrolysis reaction. As such, advancements in technology aim to make the process more energy-efficient and environmentally friendly.
4. Safety and Handling: Chlorine is a toxic and corrosive gas. During its production, transport, and use, strict safety measures must be observed to prevent exposure and environmental contamination. This includes the use of specialized equipment and protocols for handling and storage.
Understanding the uses and commercial production of chlorine allows for a greater appreciation of this element’s role in modern society, from ensuring clean drinking water to being a fundamental building block in various industries. Its versatile applications and the evolving methods of its production reflect its integral position in the chemical industry.
Sulfur in various forms has diverse implications for health, ranging from beneficial in small amounts to harmful in larger concentrations or particular compounds.
Sulfur and its compounds also have significant environmental implications, affecting air quality, climate, and ecosystems.
Understanding the health and environmental effects of sulfur is crucial for managing its use in industry, agriculture, and consumer products, as well as for regulating emissions to protect human health and the environment. This knowledge helps inform policies and personal decisions related to air quality, industrial processes, and dietary choices.
Chlorine gas can be harmful; it irritates the respiratory system, eyes, and skin. Prolonged exposure may lead to severe health issues.
Chlorine is widely used for water purification, disinfection, and in making plastics, solvents, and other chemicals.
Chlorine is banned in some applications due to its high toxicity and potential for harmful environmental and health effects.
Chlorine is a chemical element with the symbol Cl and atomic number 17, primarily extracted from salt through electrolysis.
Yes, chlorine is a primary component in bleach, commonly used as sodium hypochlorite for disinfection and whitening.
No, chlorine is a non-metal. It is a yellow-green gas at room temperature and belongs to the halogen group in the periodic table.
Chlorine is a critical element with vast applications and implications. This guide has illuminated its uses, handling, and safety tips, aiming to provide comprehensive knowledge for effective and cautious utilization. Always prioritize safety and environmental considerations to harness chlorine’s benefits while minimizing its risks, enhancing both understanding and application.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of chlorine?
16
17
18
20
Which of the following is a common use of chlorine?
Fuel additive
Water disinfection
Food preservative
Rust remover
Chlorine is a component of which of the following compounds?
Ammonia
Table salt
Baking soda
Vinegar
At room temperature, what is the physical state of chlorine?
Solid
Liquid
Gas
Plasma
What type of bond does chlorine form with sodium to create sodium chloride?
Covalent bond
Ionic bond
Metallic bond
Hydrogen bond
Which property of chlorine is utilized in swimming pools?
Flammability
Solubility in water
Radioactivity
Oxidizing ability
Chlorine belongs to which group on the periodic table?
Alkali metals
Alkaline earth metals
Halogens
Noble gases
What is the effect of chlorine exposure on human health?
It has no effect
It is beneficial in small quantities
It can cause respiratory issues
It enhances oxygen absorption
Which of the following is not a characteristic of chlorine?
It has a pungent odor
It is heavier than air
It is a good conductor of electricity
It appears yellow-green
How does chlorine react with sodium to form sodium chloride?
Sodium donates an electron to chlorine.
Sodium shares an electron with chlorine.
Chlorine donates an electron to sodium.
Chlorine shares an electron with sodium.
Before you leave, take our quick quiz to enhance your learning!

