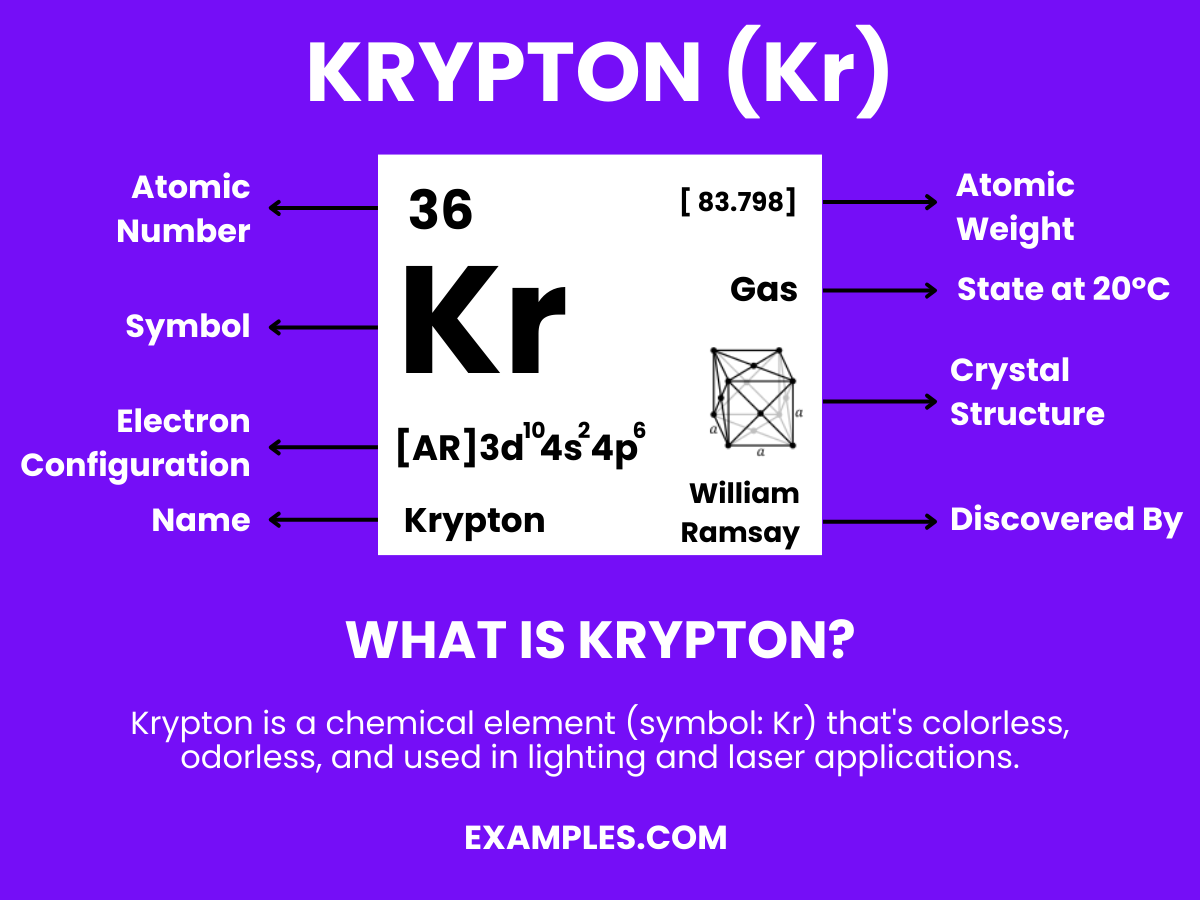
Krypton
Enter the noble world of Krypton, an element that’s not just a part of superhero lore but a real-life gas with unique properties. In this 75-word guide, teachers will find rich information and practical examples to bring the wonders of Krypton into the classroom. From its use in lighting to its role in scientific measurements, Krypton’s versatility is bound to spark curiosity and enhance learning. Let’s uncover the secrets of Krypton together, making science education more dynamic and engaging.
What is Krypton?
Krypton is a noble gas with the chemical symbol Kr and atomic number 36. It’s colorless, odorless, and tasteless, and is found in trace amounts in the Earth’s atmosphere. Krypton is known for its stability and inertness, rarely forming compounds. It’s used in various applications, including high-intensity lighting, fluorescent lamps, and as a standard in measuring the length (as the definition of a meter was once based on krypton’s spectral lines). Its rarity and unique properties make it a fascinating subject in physics and chemistry education.
Other Noble Gases
| Helium |
| Neon |
| Argon |
| Xenon |
| Radon |
Krypton Formula
- Formula: Kr
- Composition: A single krypton atom.
- Bond Type: Krypton atoms typically do not form bonds due to a complete valence shell.
- Molecular Structure: Monatomic gas.
- Electron Configuration: Eight valence electrons, with a total of 36 electrons and the configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶.
- Significance: Krypton is used in lighting, particularly in high-intensity discharge lamps and fluorescent bulbs, which emit a brilliant white light. It’s also used in some photographic flashes for high-speed photography.
- Role in Chemistry: While krypton is inert and not typically involved in chemical reactions, it does form some compounds under extreme conditions, such as krypton difluoride (KrF₂). Its role in the chemistry is mostly in the study of noble gas compounds and in applications requiring a stable, inert atmosphere.
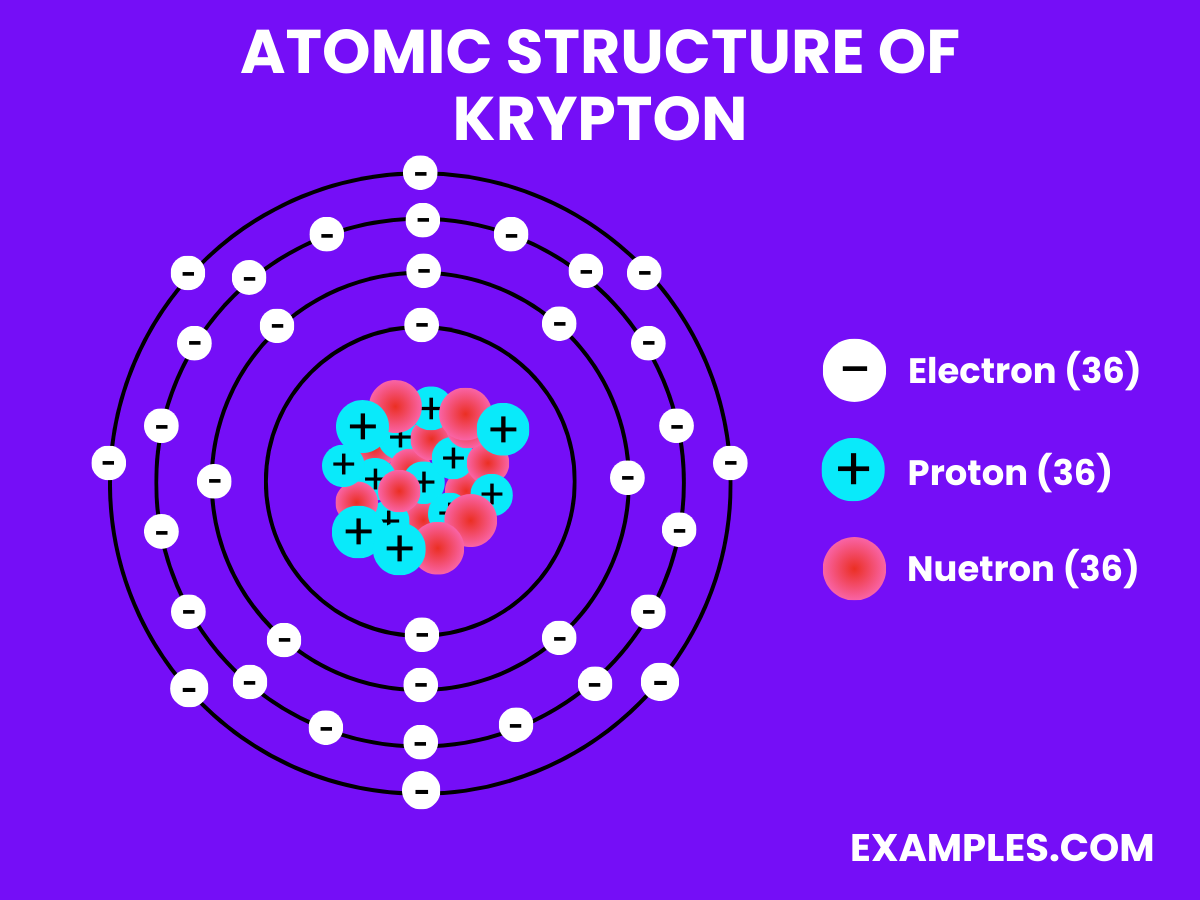
Atomic Structure of Krypton
Properties of Krypton
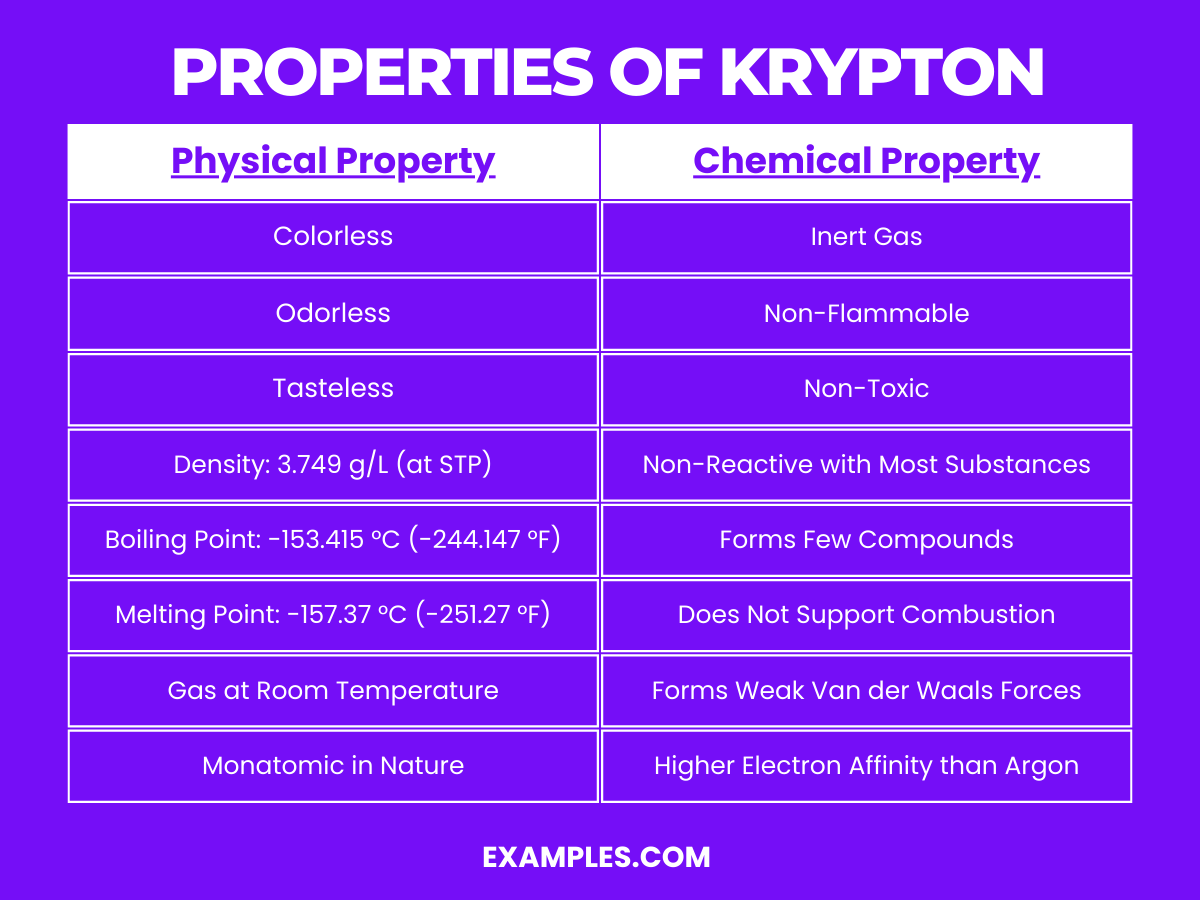
Physical Properties of Krypton
| Property | Description |
|---|---|
| State at Room Temperature | Gas |
| Color | Colorless |
| Odor | Odorless |
| Taste | Tasteless |
| Density | 3.749 g/L at 0°C and 1 atm |
| Boiling Point | -153.415 °C (-244.147 °F) |
| Melting Point | -157.37 °C (-251.27 °F) |
| Monatomic Nature | Exists as single atoms in gaseous state |
Chemical Properties of Krypton
Krypton is another noble gas, exhibiting chemical properties typical to its group, primarily characterized by low reactivity due to its complete valence electron shell:
- Inert Gas: Krypton is largely unreactive due to its full outer electron shell, similar to other noble gases. It doesn’t form many compounds and is stable in most conditions.
- Non-Flammable: As an inert gas, krypton does not burn and is not flammable, making it safe in environments where combustion is a risk.
- Non-Toxic: Krypton is non-toxic and poses no known health hazard from inhalation in small quantities. Like other inert gases, the primary risk is asphyxiation from displacement of oxygen in confined spaces.
- Forms Few Compounds: Krypton forms few compounds, and those that do exist are typically only stable under extreme conditions. One of the few known compounds is krypton difluoride (KrF₂):
- Equation: Kr+F₂→KrF₂
- This compound is a powerful oxidizing agent and is one of the most notable krypton compounds.
- Does Not Support Combustion: Due to its inertness, krypton does not support or enhance combustion, often used in applications where non-reactive environments are needed.
- Weak Van der Waals Forces: As a noble gas, krypton exhibits weak intermolecular Van der Waals forces, contributing to its physical properties like boiling and melting points.
- Higher Electron Affinity than Argon: While still low, krypton has a slightly higher electron affinity compared to lighter noble gases like helium or argon, allowing for the formation of a few more compounds.
- Existence Mostly as Atomic Gas: In its natural state, krypton exists as single atoms (monatomic) rather than molecules, which is common among the noble gases.
Thermodynamic Properties of Krypton
| Property | Description / Value |
|---|---|
| Melting Point | -157.36°C (-251.25°F) |
| Boiling Point | -153.22°C (-243.80°F) |
| Thermal Conductivity | 9.43 × 10^-3 W/(m·K) at 273K |
| Specific Heat | 0.248 J/(g·K) at 20°C |
| Heat of Vaporization | 9.08 kJ/mol at boiling point |
| Heat of Fusion | 1.64 kJ/mol at melting point |
Material Properties of Krypton
| Property | Description / Value |
|---|---|
| Phase at STP | Gas |
| Density | 3.749 g/L at 0°C, 101.325 kPa (gas) |
| Approximately 2.413 g/cm³ at -153°C (liquid) | |
| Solubility in Water | 0.114 mg/L at 20°C |
| Color | Colorless |
Electromagnetic Properties of Krypton
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Diamagnetic |
| Electrical Conductivity | Non-conductor (Krypton is a dielectric material) |
Nuclear Properties of Krypton
| Property | Description / Value |
|---|---|
| Atomic Number | 36 |
| Atomic Mass | 83.798 u |
| Neutron Cross Section | 25 barns (for ^84Kr) |
| Isotopes | Stable: ^78Kr, ^80Kr, ^82Kr, ^83Kr, ^84Kr, ^86Kr |
| Radioactive: ^81Kr, ^85Kr (and others with shorter half-lives) | |
| Radioactivity | ^85Kr (half-life: 10.76 years), ^81Kr (used in dating with a half-life of 229,000 years) |
Chemical Compounds of Krypton
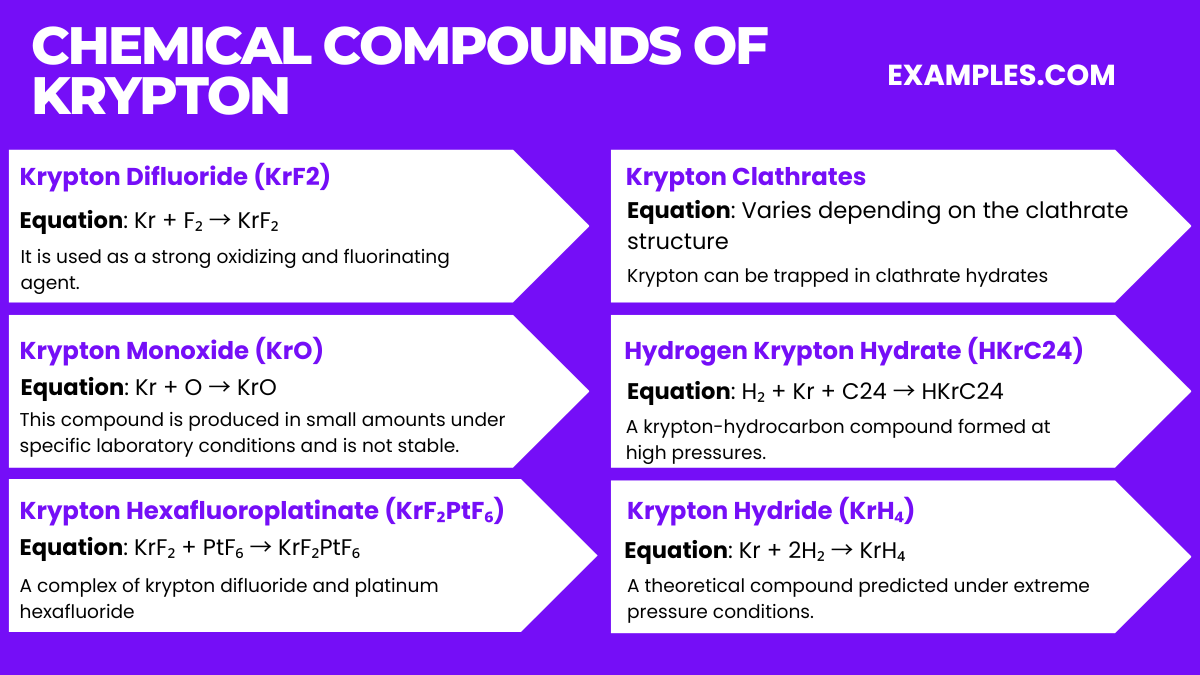
Krypton, being a noble gas, forms very few compounds, and they are generally unstable. However, here are some compounds that have been identified:
- Krypton Difluoride (KrF₂)
- Equation: Kr + F₂ → KrF₂
- It is used as a strong oxidizing and fluorinating agent.
- Krypton Monoxide (KrO)
- Equation: Kr + O → KrO
- This compound is produced in small amounts under specific laboratory conditions and is not stable.
- Krypton Hexafluoroplatinate (KrF₂PtF₆)
- Equation: KrF₂ + PtF₆ → KrF₂PtF₆
- A complex of krypton difluoride and platinum hexafluoride, it is one of the first compounds of krypton discovered.
- Krypton Clathrates
- Equation: Varies depending on the clathrate structure
- Krypton can be trapped in clathrate hydrates, where it is physically, not chemically, bound within a lattice of water molecules.
- Hydrogen Krypton Hydrate (HKrC24)
- Equation: H₂ + Kr + C24 → HKrC24
- A krypton-hydrocarbon compound formed at high pressures.
- Krypton Hydride (KrH4)
- Equation: Kr + 2H₂ → KrH₄
- A theoretical compound predicted under extreme pressure conditions.
Isotopes of Krypton
| Isotope | Natural Abundance | Atomic Mass | Half-Life | Description |
|---|---|---|---|---|
| Krypton-78 | Trace | 77.9204 u | Stable | Trace isotope found in the atmosphere. |
| Krypton-80 | 2.28% | 79.9164 u | Stable | One of the stable isotopes, used in the study of atmospheric krypton. |
| Krypton-82 | 11.58% | 81.9135 u | Stable | The most abundant stable isotope of krypton. |
| Krypton-83 | 11.49% | 82.9141 u | Stable | Used in magnetic resonance imaging (MRI) for imaging airways and lungs. |
| Krypton-84 | 57% | 83.9115 u | Stable | The second most abundant stable isotope of krypton. |
| Krypton-85 | Trace (radioactive) | 84.9125 u | 10.756 years | A byproduct of nuclear fission with applications in leak detection and atmospheric studies. |
| Krypton-86 | 17.3% | 85.9106 u | Stable | Used to define the standard meter from 1960 to 1983, as the wavelength of light from krypton-86 isotope was used. |
Uses of Krypton
- Lighting: Krypton is used in various lighting products, including fluorescent bulbs and airport runway lights. It provides an efficient light source, with a characteristic bright white light, and extends the life of the bulbs.
- Windows Insulation: Krypton gas is used in double or triple glazed window units as an insulating material between panes. Its low thermal conductivity makes it an excellent insulator, reducing heat transfer and improving energy efficiency in buildings.
- Photography and High-Speed Photography: The intense white light produced by krypton flashes is used in high-speed photography to capture rapid sequences of events, providing clear, bright illumination.
- MRI Imaging in Medicine: The isotope Krypton-83 has applications in magnetic resonance imaging (MRI), particularly in the imaging of airways and lungs. Its magnetic properties make it useful as an inhalable gas in respiratory studies.
- Leak Detection: The radioactive isotope Krypton-85 is used in the detection of leaks in sealed containers and systems. Its ability to penetrate small openings and be detected by radiation monitors makes it useful in quality assurance and safety checks.
- Aerospace and Research: Krypton is utilized in various aerospace and research applications, including propulsion systems for space crafts and as a standard for measuring certain physical properties due to its stable and well-defined spectral lines.
Commercial Production of Krypton
Krypton is commercially produced through the fractional distillation of liquid air. This process involves the cooling of air to a point where it liquefies, followed by gradual warming that allows the separate gases to boil off at their respective boiling points. Here are the key steps:
- Air Liquefaction: Air is first cooled to very low temperatures to liquefy it. This is typically achieved through processes involving compression and expansion cycles.
- Fractional Distillation: The liquefied air is then subjected to fractional distillation in a distillation column. As the liquid mixture is slowly heated, different gases boil off at their specific boiling points and are collected at different levels of the column. Argon, neon, krypton, and xenon are collected as they have higher boiling points than nitrogen and oxygen.
- Further Purification: After the initial separation, krypton often requires further purification to remove any remaining gases or impurities. This can involve additional distillation steps, adsorption methods, or chemical reactions, depending on the desired purity level.
- Storage and Transportation: Once purified, krypton is usually stored and transported in high-pressure cylinders, ensuring that it remains in a gaseous state and is ready for use in various applications.
Health Effects of Krypton
Krypton is a noble gas that is generally non-toxic and chemically inert. However, it can have specific health effects under certain conditions:
- Asphyxiation: Like other inert gases, the primary health risk associated with krypton is asphyxiation. In high concentrations, especially in enclosed or poorly ventilated areas, krypton can displace oxygen in the air, leading to suffocation. Symptoms of asphyxiation might include dizziness, breathlessness, and loss of consciousness.
- Pressure Effects: If krypton gas is released rapidly, particularly in a confined space, it can create a high-pressure environment that could lead to physical injuries or damage the respiratory system due to the rapid expansion or contraction of air.
- Minimal Direct Toxicity: Krypton is considered to have minimal direct toxicity due to its inert nature. It doesn’t react chemically with body tissues or interfere with biological processes at typical exposure levels.
Safety measures, such as adequate ventilation and the use of appropriate protective equipment, are crucial when working with krypton in industrial or laboratory settings to mitigate the risks associated with its physical properties.
Environmental Effects of Krypton
Krypton is a naturally occurring element in the Earth’s atmosphere and is present in trace amounts. Here are its environmental effects:
- Inert Nature: Krypton’s most significant environmental feature is its chemical inertness. It does not react with other elements or compounds in the environment under normal conditions. This means it does not contribute to chemical pollution, participate in atmospheric reactions, or affect water or soil quality.
- No Known Significant Environmental Impact: There are no known significant ecological roles or negative environmental impacts attributed to krypton due to its stability and inert nature. It does not participate in biological processes, and there is no evidence that it affects ecosystems or organisms.
- Atmospheric Presence: Krypton exists in the atmosphere at very low concentrations (about 1 part per million). Its presence does not contribute to greenhouse gases or other atmospheric phenomena that affect climate change.
How is Krypton Harmful to Humans?
Krypton is largely non-toxic but can cause asphyxiation by displacing oxygen in enclosed spaces, leading to dizziness or unconsciousness.
What are 4 Interesting Facts about Krypton?
- Krypton lights up with a brilliant white light.
- It’s used in high-speed photography.
- Constitutes about 1 ppm of Earth’s atmosphere.
- Krypton-83m is used in MRI imaging.
Why is Krypton So Cool?
Krypton is cool due to its rare, inert nature, brilliant light emission in bulbs, and unique applications in high-tech fields like laser technology and photography.
Why Krypton is Called Hidden Gas?
Krypton is called a hidden gas due to its rare and inert nature, derived from the Greek word “kryptos,” meaning hidden, reflecting its elusive presence.
What is Krypton Used For?
Krypton is used in lighting (fluorescent bulbs), insulation for windows, high-speed photography, and MRI lung imaging, capitalizing on its inert properties.
Krypton is a noble gas with unique applications, from lighting to high-speed photography. Its inertness ensures safety and effectiveness in various industries. Understanding Krypton’s properties, safe handling, and innovative uses is essential for its optimal application. Stay informed and cautious to utilize Krypton’s benefits efficiently and safely in your endeavors.