What is the most common allotrope of oxygen in Earth's atmosphere?
Ozone (O3)
Atomic oxygen (O)
Molecular oxygen (O2)
Oxygen difluoride (OF2)

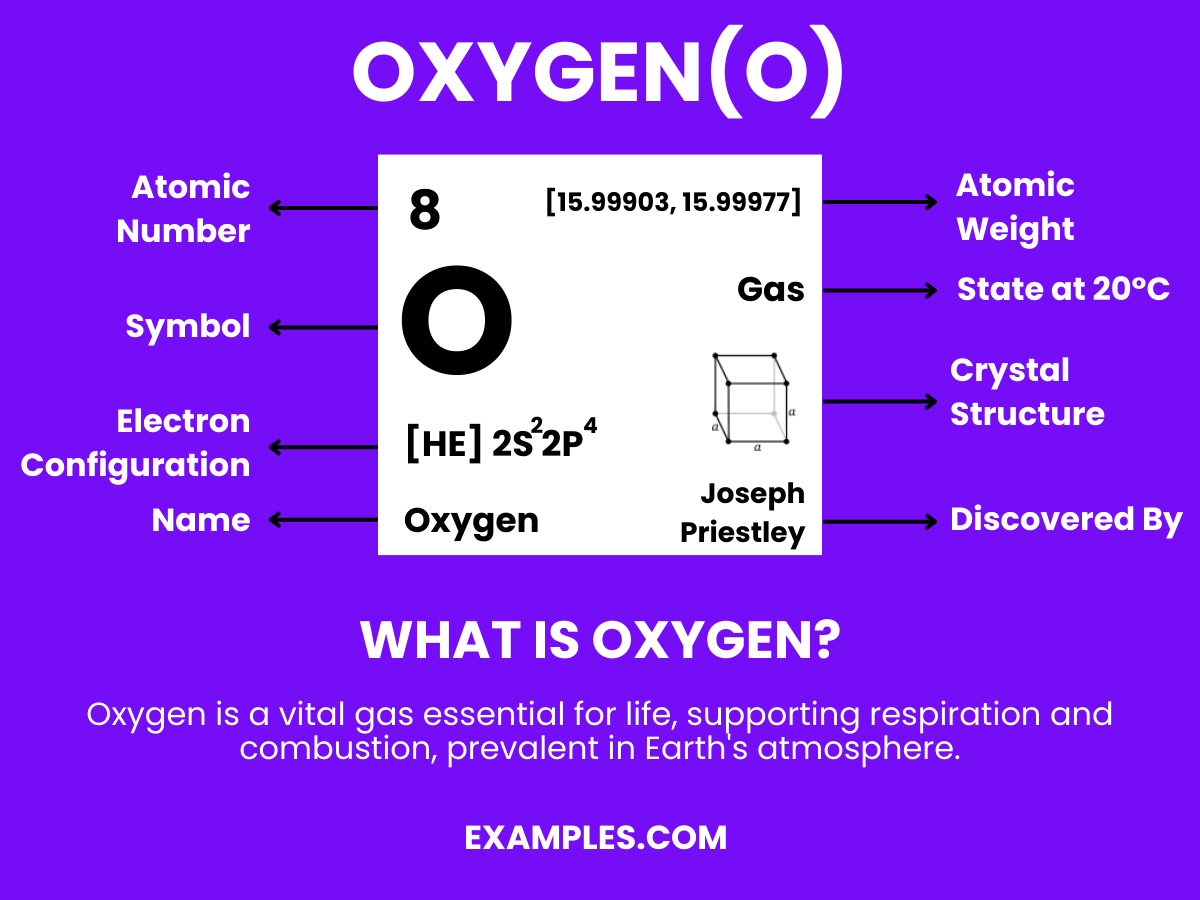
Oxygen is the lifeblood of our planet, essential for combustion, respiration, and countless other processes. In this comprehensive guide, we delve into the fascinating world of oxygen, its interactions, and its vital partnership with hydrogen in creating water. Understand the nuances of this element through vivid examples, ensuring a clear understanding of its paramount importance in both the microscopic world of molecules and the vast expanse of ecosystems.
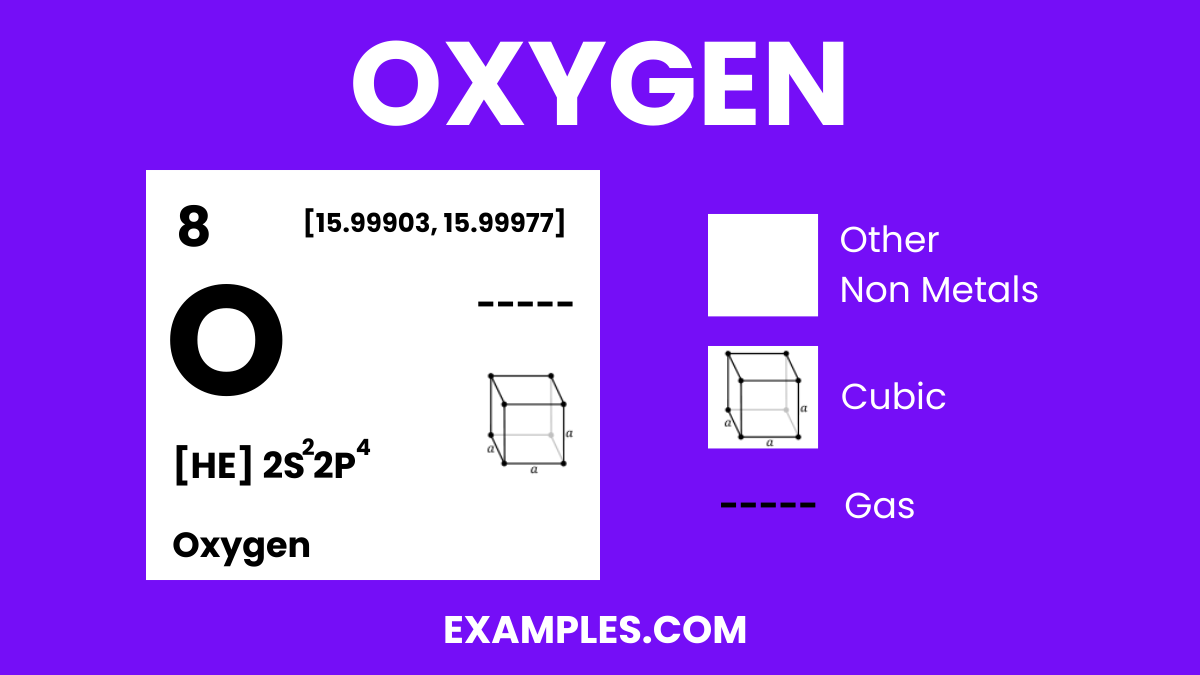
Oxygen is a colorless, odorless, and tasteless gas that is essential for life on Earth. It is the third most abundant element in the universe and makes up about 21% of the Earth’s atmosphere. Oxygen is crucial for respiration in living organisms and is used in various industrial applications. It combines with most elements, forms oxides, and is a part of water molecules when it reacts with hydrogen. Understanding oxygen is fundamental in fields ranging from environmental science to human physiology.
| Hydrogen | Sulfur |
| Carbon | Chlorine |
| Nitrogen | Selenium |
| Fluorine | Bromine |
| Phosphorus | Iodine |
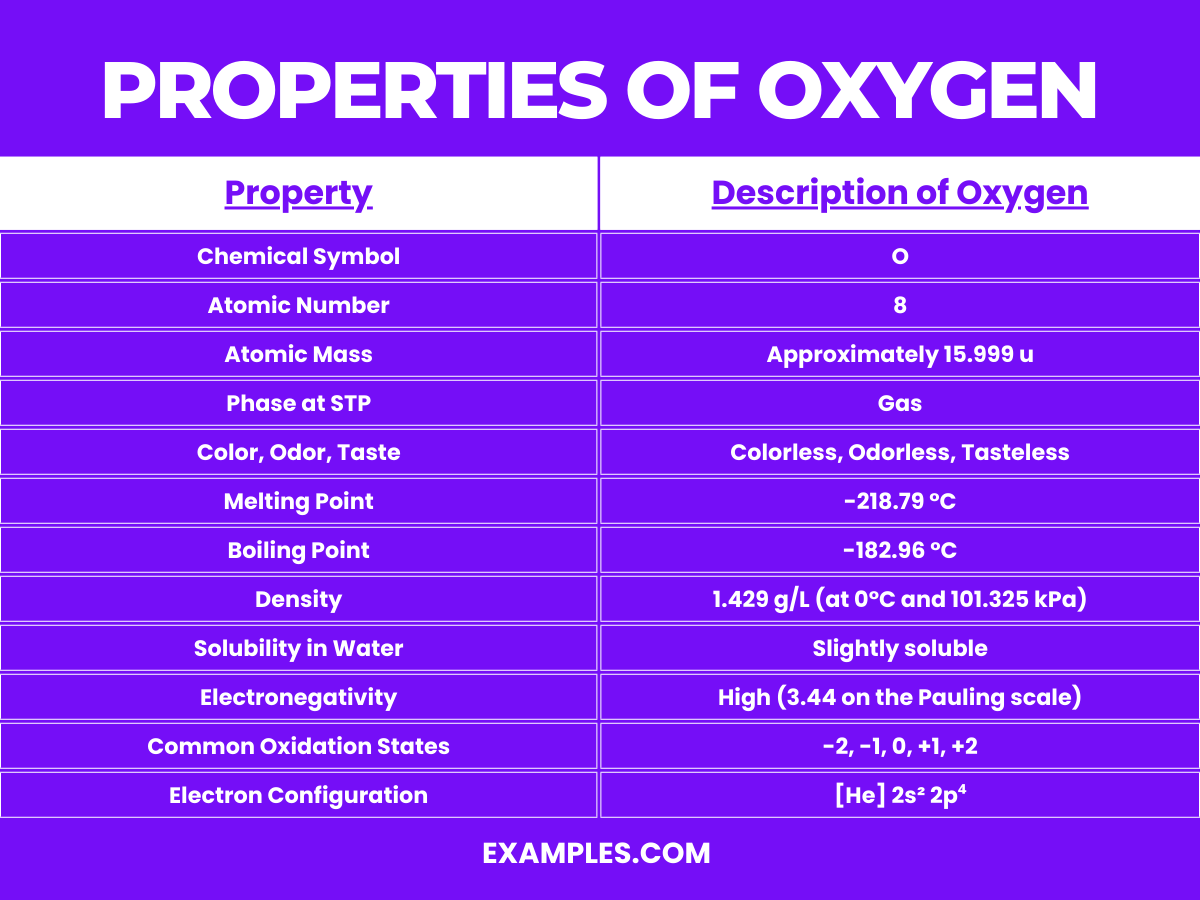
Oxygen, denoted by the chemical symbol O, is a fundamental element known for its life-sustaining properties. Here are the detailed physical properties of oxygen:
Understanding these physical properties is crucial for utilizing oxygen in various industrial, medical, and scientific applications. From supporting life through respiration to its use in steelmaking and rocket propulsion, oxygen’s physical properties underpin its wide range of applications.
Oxygen is a highly reactive non-metallic element and a member of the chalcogen group on the periodic table. It is essential for life and participates in various chemical reactions. Here are some of the detailed chemical properties of oxygen:
| Property | Description / Value |
|---|---|
| Melting Point | -218.79°C (-361.82°F) |
| Boiling Point | -182.96°C (-297.33°F) |
| Thermal Conductivity | 0.02658 W/(m·K) at 300K |
| Specific Heat | 0.918 J/(g·K) at 298K |
| Heat of Vaporization | 6.82 kJ/mol at boiling point |
| Heat of Fusion | 0.444 kJ/mol at melting point |
| Property | Description / Value |
|---|---|
| Phase at STP | Gas |
| Density | 1.429 g/L at 0°C, 101.325 kPa (gas) |
| 1.141 g/cm³ at -183°C (liquid) | |
| Solubility in Water | 30.8 mg/L at 20°C and 1 atm |
| Color | Pale blue (in its liquid and solid form) |
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Paramagnetic (strongly attracted to magnets) |
| Electrical Conductivity | Non-conductor (oxygen is a dielectric material) |
| Property | Description / Value |
|---|---|
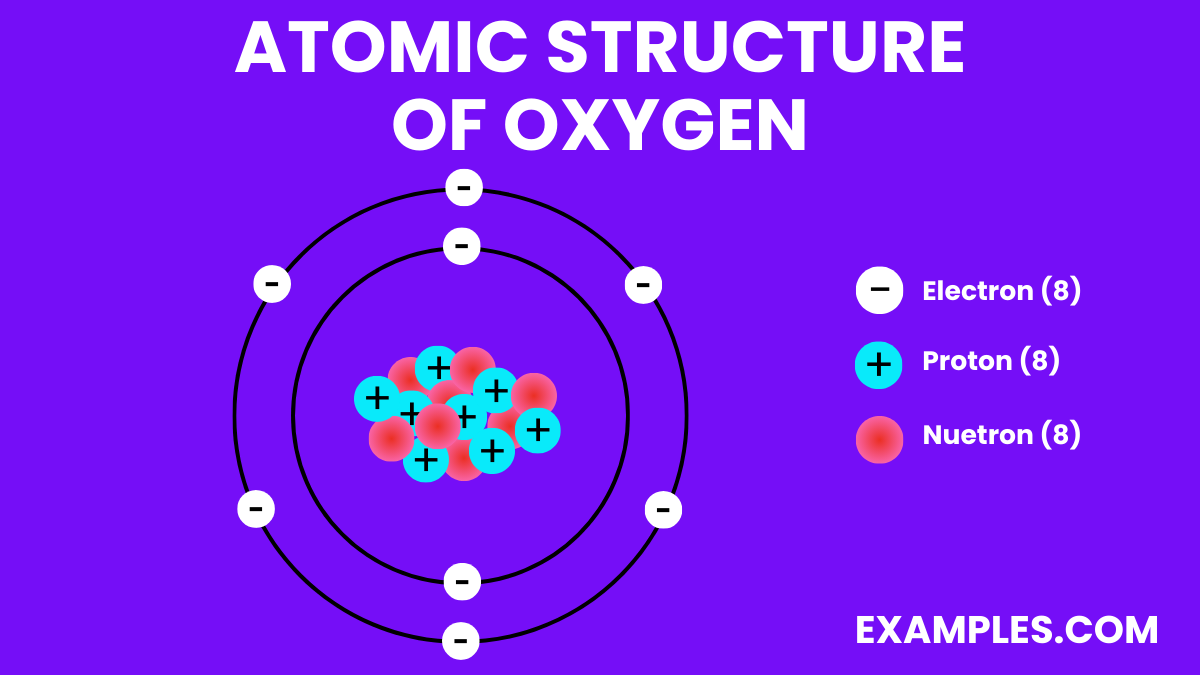
| Atomic Number | 8 |
| Atomic Mass | 15.999 u |
| Neutron Cross Section | 0.00019 barns (for ¹⁶⁰) |
| Isotopes | ¹⁶⁰ (99.76%), ¹⁷⁰ (0.038%), ¹⁸⁰ (0.20%) |
| Radioactivity | Oxygen has no naturally occurring radioactive isotopes. ^15O is a radioactive isotope used in medical diagnostics, with a half-life of 122.24 seconds |

Oxygen is a vital element essential for life and various industrial processes. Understanding its preparative methods is crucial for both academic and practical applications. Here we delve into the common methods used to prepare oxygen, providing relevant equations to illustrate the chemical processes involved.
One of the primary methods for obtaining pure oxygen is through the fractional distillation of liquified air. Air is cooled to very low temperatures to liquefy it. Due to the different boiling points of nitrogen (-195.8°C) and oxygen (-183.0°C), oxygen can be separated as it boils off after nitrogen.
Equation: Air (Liquified)→N₂(g)+O₂(g)+other gases
Electrolysis of water is a common laboratory method for producing oxygen. By applying an electric current to water, it decomposes into oxygen and hydrogen gas.
Equation: 2H₂O(l)→electriccurrent2H₂(g)+O₂(g)
Certain chemical compounds release oxygen when heated or treated with other chemicals. Examples include potassium chlorate (KClO₃), which releases oxygen upon heating in the presence of a catalyst like manganese dioxide (MnO₂).
Equation for Potassium Chlorate Decomposition: 2KClO₃→MnO₂2KCl+3O₂(g)
While not a method to industrially produce oxygen, it’s vital to understand that photosynthesis in plants is a natural source of atmospheric oxygen. Green plants convert carbon dioxide and water into glucose and oxygen in the presence of sunlight.
Equation: 6CO₂+6H₂O+ light energy→C6H₁₂O₆+6O₂
Understanding these preparative methods of oxygen is crucial for various applications, including medical uses, industrial processes, and environmental management. Each method has its advantages and applications, contributing to our utilization and appreciation of this essential element.
Oxygen forms a wide array of compounds, including oxides, acids, and others. Here are some of the significant types of oxygen compounds:
Almost all elements can combine with oxygen to form oxides. These compounds typically have oxygen in a -2 oxidation state. They can be further classified into acidic, basic, or amphoteric oxides depending on their properties.
Equations:
Peroxides contain an O-O single bond with each oxygen typically having a -1 oxidation state. Superoxides, usually formed with alkali metals, have even more reduced oxygen.
Equations:
Many oxygen-containing acids are fundamental in chemistry and biology, including sulfuric acid (H₂SO₄), nitric acid (HNO₃), and carbonic acid (H₂CO₃).
Equations:
Oxygen is also a constituent of esters and ethers, which are organic compounds widely used in industry and biology.
Equations:
Each of these compounds demonstrates the versatility and reactivity of oxygen, making it an indispensable element in countless chemical reactions and applications. Understanding the preparative methods and chemical compounds of oxygen offers a foundation for exploring its critical role in both nature and industry. This guide not only serves as an educational resource but also underscores the importance of oxygen in various fields, from healthcare to environmental science.
Oxygen has several isotopes, with Oxygen-16 (16O), Oxygen-17 (17O), and Oxygen-18 (18O) being the most naturally abundant and stable. Below is a table describing these isotopes:
| Isotope | Atomic Mass | Abundance | Stability | Notable Features |
|---|---|---|---|---|
| Oxygen-16 (16O) | 15.994 u | 99.762% | Stable | Most abundant, involved in the water cycle and used as a standard for δ18O paleoclimatology studies. |
| Oxygen-17 (17O) | 16.999 u | 0.038% | Stable | Rare, used in respiratory studies in medical applications to trace oxygen molecules. |
| Oxygen-18 (18O) | 17.999 u | 0.200% | Stable | Used in paleoclimate reconstruction and as a tracer in earth sciences and medical diagnostics. |
Oxygen is a versatile element, playing a critical role in various processes and industries. Here are the major uses of oxygen:
Healthcare: Oxygen is widely used in medical treatments, including oxygen therapy for patients with respiratory issues.
2. Steel Manufacturing: In steel plants, oxygen is used to increase the efficiency of the burning process in blast furnaces.
3. Chemical Industry: Oxygen is a key reactant in many industrial chemical reactions, including the production of ethylene oxide and synthesis gas.
4. Water Treatment: Oxygen is used in the treatment of sewage and industrial effluents by facilitating the aerobic digestion of pollutants.
5. Aerospace & Submarine Life Support: Pure oxygen is used in space missions and submarines to support life in enclosed spaces.
Oxygen, a vital element for life, has numerous health effects on the human body. Its proper balance is essential for maintaining overall health and well-being.
Oxygen, while essential for life and various ecological functions, also has significant environmental impacts.
Understanding the comprehensive roles and effects of oxygen is crucial for both human health and environmental sustainability. With its ubiquitous presence and involvement in critical processes, oxygen remains a focal point in scientific and medical research, as well as environmental management.
Oxygen (O) refers to the element, while O₂ is diatomic oxygen, the common, stable form of oxygen gas in the Earth’s atmosphere.
Oxygen is used for respiration, medical treatments, water treatment, and as an industrial oxidizing agent.
Oxygen is made up of two oxygen atoms bonded together (O₂) in its most stable form, featuring a double bond.
O₂ is not a compound but a diatomic molecule, as it consists of two atoms of the same element, oxygen.
Understanding oxygen is pivotal in various domains from health to environmental science. This guide has illuminated oxygen’s properties, uses, and significance. Embracing these insights and tips will enhance your comprehension and application of oxygen, fostering a more informed perspective on this essential element that profoundly influences both life and the environment.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the most common allotrope of oxygen in Earth's atmosphere?
Ozone (O3)
Atomic oxygen (O)
Molecular oxygen (O2)
Oxygen difluoride (OF2)
Oxygen is essential for the process of:
Photosynthesis
Fermentation
Nitrification
Polymerization
Which layer of the atmosphere contains the highest concentration of ozone?
Troposphere
Stratosphere
Mesosphere
Thermosphere
What is the primary role of oxygen in cellular respiration?
To break down glucose molecules
To convert ADP to ATP
To act as a final electron acceptor
To synthesize protein
Oxygen was first discovered by which scientist?
Antoine Lavoisier
Joseph Priestley
Carl Wilhelm Scheele
Both B and C
What percentage of the Earth's atmosphere is made up of oxygen?
Approximately 21%
Approximately 78%
Approximately 10%
Approximately 35%
Oxygen toxicity primarily affects which organ?
Heart
Lungs
Liver
Kidneys
Which industrial process is used to produce oxygen from air?
Electrolysis
Fractional distillation
Chemical synthesis
Catalytic conversion
What property of oxygen supports combustion?
It is a reductant
It is a catalyst
It is a reactant
It is an inhibitor
In water (H2O), oxygen has a formal oxidation state of:
-1
-2
0
+1
Before you leave, take our quick quiz to enhance your learning!

