What is the atomic number of Erbium?
66
67
68
69
The comprehensive guide on Erbium, a pivotal element in the world of science and technology. This article offers an insightful exploration of Erbium, detailing its definition, intrinsic properties, and the multitude of its applications ranging from telecommunications to medical equipment. Through illustrative examples, you’ll discover why Erbium is not just another element but a cornerstone in advancing modern innovations. Enhance your understanding of how Erbium’s unique characteristics and compounds contribute to its critical role across various industries.
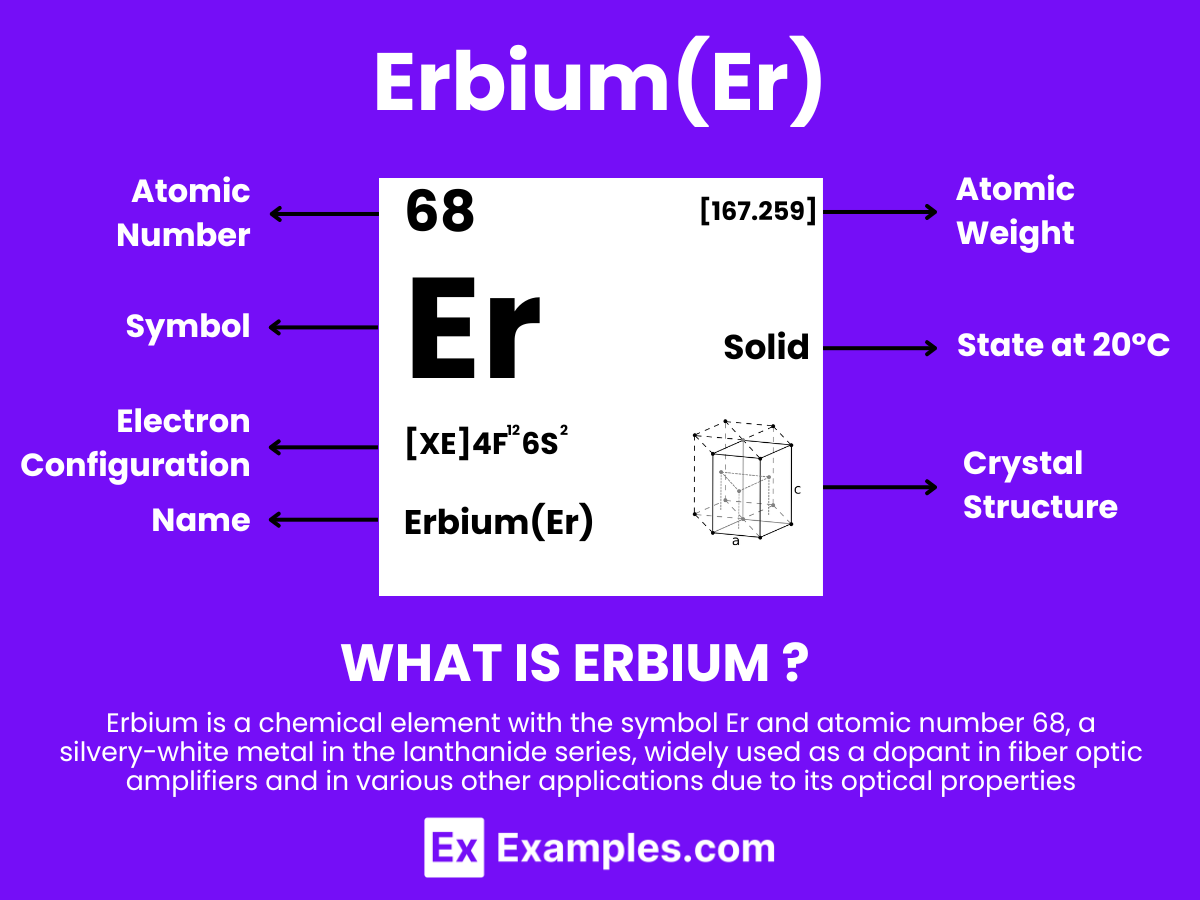
Erbium is a silvery-white metallic element belonging to the lanthanide series of the periodic table, with the atomic number 68. Known for its pink-colored ions, erbium finds extensive use in optical fiber amplifiers, lasers, and in the coloring of glasses and ceramics. Its natural occurrence is mainly found in minerals like xenotime and euxenite, often extracted through complex chemical processes. Erbium’s unique properties, such as its ability to absorb infrared light, make it an indispensable component in modern telecommunications, offering the potential for significant advancements in technology and materials science.
Erbium, in contrast to hydrogen, is a metallic element with characteristics that reflect its status as a member of the lanthanide series of the periodic table. Its properties include a metallic luster, malleability, and conductivity, which significantly diverge from the gaseous nature and simple molecular formation of hydrogen. Erbium’s behavior at the atomic and molecular levels is defined by its electronic structure and its role in various chemical and physical processes.
Atomic Level: Each erbium atom (Er) contains 68 protons in its nucleus and is expected to have 68 electrons orbiting around it. The electron configuration of erbium is predicted to be [Xe] 4f¹² 6s², indicating a complex electron configuration with potential for various oxidation states, similar to other elements in the lanthanide series. This suggests a certain level of chemical reactivity, allowing erbium to form compounds with other elements. The partially filled 4f electron shell of erbium contributes to its unique optical and magnetic properties.
Molecular Formation: Unlike hydrogen, which forms simple diatomic molecules (H₂) through covalent bonding, erbium would not form molecules in a similar manner due to its metallic nature. In bulk form, erbium is expected to exhibit a metallic lattice structure typical of metals. This structure involves metallic bonding, where electrons are delocalized over many erbium atoms, differing fundamentally from the discrete electron sharing seen in hydrogen’s covalent bonds. The crystalline structure of solid erbium is usually hexagonal close-packed (hcp), which is common among rare earth metals.
| Physical Properties | Chemical Properties |
|---|---|
| Atomic Number: 68 | Oxidation States: +3 |
| Atomic Mass: 167.259 g/mol | Electronegativity: 1.24 (Pauling scale) |
| Density: 9.066 g/cm³ at 20°C | Reactivity with Water: Slow to react, forming erbia (Er₂O₃) |
| Melting Point: 1529°C | Common Ions: Er³⁺ |
| Boiling Point: 2868°C | Reactivity with Air: Forms oxide layer upon exposure |
| State at Room Temperature: Solid | Stability: Resistant to corrosion, but oxidizes in air |
| Thermal Conductivity: 14.5 W/(m·K) | Acid Solubility: Soluble in mineral acids |
| Electrical Resistivity: 0.86 microohm·m at 20°C | Affinity for Oxygen: High, forms stable oxides |
| Property | Value |
|---|---|
| Appearance | Silvery-white, soft metal |
| Atomic Mass | 167.259 g/mol |
| Melting Point | 1529 °C |
| Boiling Point | 2868 °C |
| Density | 9.066 g/cm³ at 20 °C |
| State at 20 °C | Solid |
| Electrical Conductivity | Good conductor |
| Thermal Conductivity | 14.5 W/(m·K) at 25 °C |
| Magnetic Ordering | Paramagnetic |
Erbium, like other lanthanides, has interesting chemical properties due to its electron configuration and its position in the periodic table. It tends to form compounds in the +3 oxidation state, which is common among the rare earth elements.
Examples of Chemical Properties and Equations:
| Property | Value |
|---|---|
| Standard Molar Entropy (S°298) | 62.9 J/(mol·K) |
| Heat of Fusion | 19.90 kJ/mol |
| Heat of Vaporization | 280 kJ/mol |
| Standard Enthalpy of Formation (ΔHf°298) | -165.4 kJ/mol for Er_2O_3 |
| Property | Value |
|---|---|
| Crystal Structure | Hexagonal Close-Packed (hcp) |
| Hardness | Soft metal, Mohs hardness ~2.5 |
| Elastic Modulus | 69.9 GPa |
| Poisson’s Ratio | 0.237 |
| Thermal Expansion Coefficient | 12.2 µm/(m·K) at 25 °C |
| Property | Description |
|---|---|
| Magnetic Ordering | Paramagnetic at room temperature |
| Electrical Resistivity | 0.86 microohm·m at 20°C |
| Thermal Conductivity | 14.5 W/(m·K) |
| Optical Properties | High absorption in visible, infrared, and near-infrared; used in optical fibers |
| Magnetic Susceptibility | Strongly paramagnetic over a wide temperature range |
| Laser Activity | Erbium-doped fibers used in lasers for their efficient emission in the 1.55 µm range |
| Property | Description |
|---|---|
| Natural Isotopes | Er-162, Er-164, Er-166, Er-167, Er-168, Er-170 |
| Most Stable Isotope | Er-166 (stable) |
| Neutron Cross Section | Moderate, varies by isotope |
| Neutron Absorption | Used in nuclear technology for its neutron absorption capabilities |
| Isotopic Abundance in Nature | Er-166 is the most abundant isotope |
| Radioactive Isotopes | Several, including Er-169 used in medicine for its gamma radiation |
Erbium is typically prepared through complex extraction and reduction processes from minerals like xenotime and monazite, which contain a mixture of rare earth elements. The preparation involves several steps:
| Isotope | Mass Number | Half-life | Primary Decay Mode |
|---|---|---|---|
| Er-162 | 162 | Stable | – |
| Er-164 | 164 | Stable | – |
| Er-166 | 166 | Stable | – |
| Er-167 | 167 | Stable | – |
| Er-168 | 168 | Stable | – |
| Er-169 | 169 | 9.4 days | Beta decay |
| Er-170 | 170 | Stable | – |
| Er-172 | 172 | Stable | – |
The production of erbium is a detailed process that involves several stages, from mining rare earth minerals to isolating and refining the erbium metal. Erbium is typically extracted from minerals such as xenotime and monazite, which contain a mix of rare earth elements. The key steps in erbium production include:
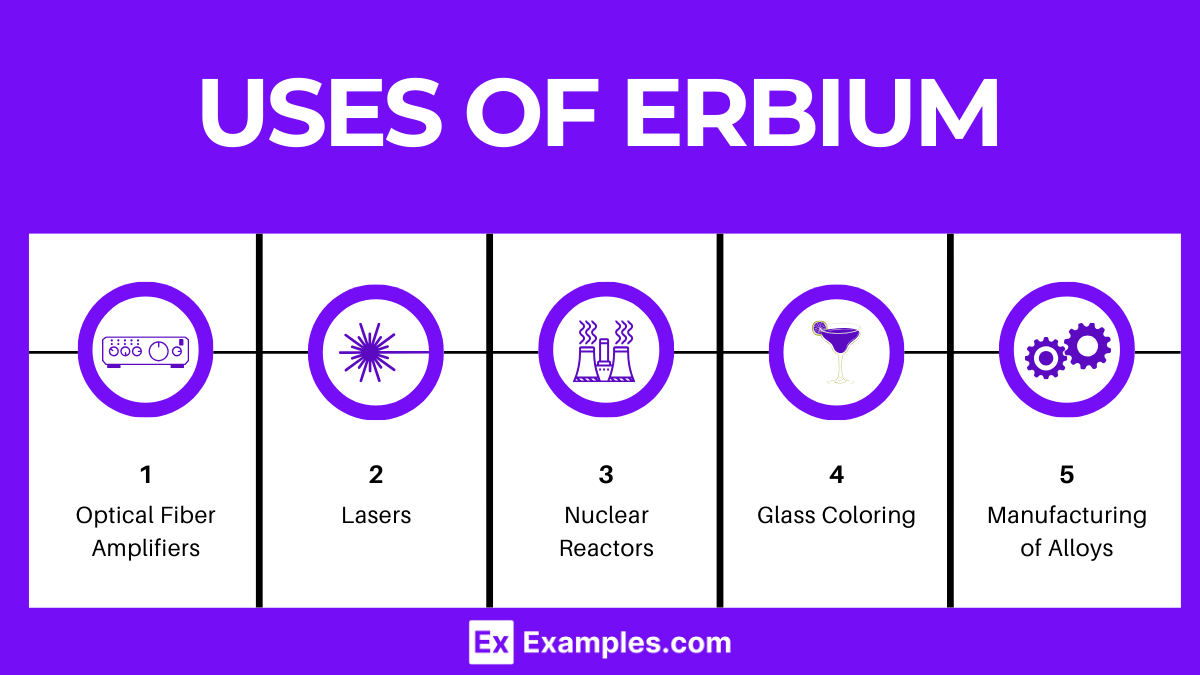
Erbium has a variety of applications across different fields, leveraging its unique properties, especially its optical characteristics. Some of the primary applications include:
This article meticulously explores erbium’s journey from production to its pivotal applications, unveiling the element’s electromagnetic and nuclear properties. Erbium’s role in enhancing telecommunications and medical technologies exemplifies its importance. By delving into its unique characteristics, the article illuminates erbium’s contribution to advancing modern technological landscapes, underscoring the element’s indispensable value in science and industry.
Text prompt
Add Tone
Isotopes of Erbium
Chemical Compounds of Erbium
What is the atomic number of Erbium?
66
67
68
69
What is the chemical symbol for Erbium?
Er
Eb
Eu
Es
Erbium belongs to which group in the periodic table?
Alkali metals
Transition metals
Lanthanides
Actinides
Which color does Erbium emit when used in laser applications?
Red
Green
Blue
Pink
What is a common use for Erbium in the telecommunications industry?
Semiconductors
Fiber optics
Batteries
Insulators
Erbium is primarily obtained from which type of mineral?
Bauxite
Monazite
Hematite
Galena
What is the melting point of Erbium?
824°C
1140°C
1529°C
1802°C
Erbium has how many naturally occurring isotopes?
4
6
8
10
Which of the following is a key property of Erbium?
Highly reactive with water
Highly reactive with water
Highly volatile
Non-conductive
In what form is Erbium commonly used in metallurgical applications?
Pure metal
Alloy
Compound
Powder
Before you leave, take our quick quiz to enhance your learning!

