What is the atomic number of Europium?
63
64
65
66
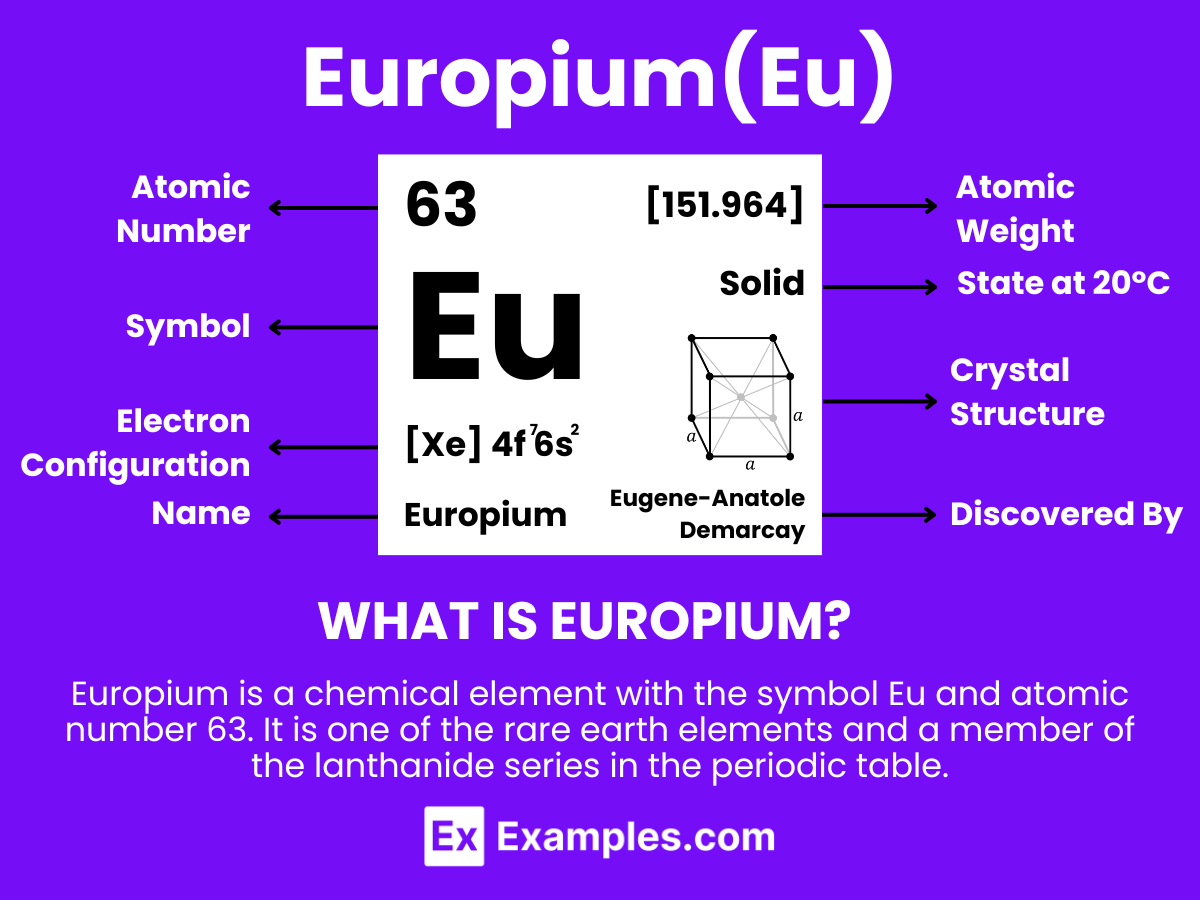
Dive into the fascinating world of Europium, an essential rare earth element that powers innovations across various industries. Our comprehensive guide illuminates Europium’s unique properties, practical applications, and its pivotal role in modern technology and materials science. Through detailed examples, explore how Europium enhances color displays, strengthens security features, and contributes to research advancements. Perfect for enthusiasts and professionals alike.
Europium is a chemical element with the symbol Eu and atomic number 63. It is one of the rare earth elements and a member of the lanthanide series in the periodic table. Europium is a silvery-white metal at room temperature, known for its high reactivity, especially against water and air, where it rapidly oxidizes. This element has two dominant oxidation states, +2 and +3, with the +3 being the most stable and common in its compounds.Europium stands out for its remarkable luminescent properties, making it widely used in the phosphors that create the red color in television and computer screens, LEDs, and other luminescent materials. Its compounds, such as europium oxide (Eu2O3), are crucial in manufacturing various consumer electronics because of their ability to absorb and emit light in a highly efficient manner.
Europium is a chemical element with the symbol Eu and atomic number 63. It belongs to the lanthanide series of the periodic table, which is characterized by the filling of the 4f electron shell. The atomic structure of Europium is key to understanding its unique properties, especially its luminescence. Below is a detailed overview of Europium’s atomic structure:
| Physical Property | Value | Units |
|---|---|---|
| Appearance | Silvery-white, tarnishes quickly in air | – |
| Atomic Number | 63 | – |
| Atomic Weight | 151.96 | Atomic Mass Units (amu) |
| Density | 5.244 | grams per cubic centimeter (g/cm³) |
| Melting Point | 1099 | Kelvin (K) |
| Boiling Point | 1802 | Kelvin (K) |
| Heat Capacity | 27.66 | Joules per mole Kelvin (J/mol·K) |
| Thermal Conductivity | 13.9 | Watts per meter Kelvin (W/(m·K)) |
| Crystal Structure | Body-centered cubic (bcc) | – |
Europium, symbolized as Eu and atomic number 63, stands out among the lanthanides due to its unique chemical properties. Here’s a detailed look into Europium’s chemical behavior:
| Property | Value | Units |
|---|---|---|
| Melting Point | 1099 K | Kelvin |
| Boiling Point | 1802 K | Kelvin |
| Heat of Fusion | 9.21 kJ/mol | Kilojoules per mole |
| Heat of Vaporization | 176 kJ/mol | Kilojoules per mole |
| Specific Heat Capacity | 27.66 J/(mol·K) | Joules per mole Kelvin |
| Thermal Conductivity | 13.9 W/(m·K) | Watts per meter Kelvin |
| Property | Value | Units |
|---|---|---|
| Density | 5.244 g/cm³ | Grams per cubic centimeter |
| Mohs Hardness | ~2.5 | Scale |
| Young’s Modulus | Approx. 18.2 GPa | Gigapascals |
| Poisson’s Ratio | Estimated < 0.3 | Dimensionless |
| Crystal Structure | Body-centered cubic | – |
| State at Room Temperature | Solid | – |
| Property | Value | Units |
|---|---|---|
| Electrical Resistivity | 0.900 µΩ·m (at 25 °C) | Microohm meters |
| Magnetic Ordering | Paramagnetic at 300 K | – |
| Curie Temperature | Not applicable | Kelvin |
| Magnetic Moment | High for Eu²⁺ ions | Bohr magnetons |
| Property | Value | Units |
|---|---|---|
| Natural Isotopes | ¹⁵¹Eu, ¹⁵³Eu | – |
| Half-life of ¹⁵¹Eu | Stable | – |
| Half-life of ¹⁵³Eu | Stable | – |
| Neutron Cross Section | ¹⁵¹Eu: 4600 barns (very high) | Barns |
| Isotopic Abundance | ¹⁵¹Eu: 47.8%, ¹⁵³Eu: 52.2% | Percent |
Conversion to Europium Oxide: The separated Europium is often initially converted into Europium oxide (Eu₂O₃) for further processing.
Metallic Reduction: Europium oxide is then reduced to metallic Europium using a reducing agent such as lanthanum metal or calcium in a high-temperature process. A common equation for this reduction is: Eu2O3+3Ca→2Eu+3CaO
1.Europium Oxide (Eu₂O₃)
2.Europium Chloride (EuCl₃)
3.Europium Fluoride (EuF₃)
4.Europium Nitrate (Eu(NO₃)₃)
5.Europium Sulfide (EuS)
6.Europium Bromide (EuBr₃)
| Isotope | Mass Number | Half-Life | Mode of Decay | Application/Significance |
|---|---|---|---|---|
| ¹³⁹Eu | 139 | 17.9 seconds | β⁺ decay (positron emission) | Research purposes |
| ¹⁴¹Eu | 141 | 40.7 seconds | β⁺ decay (positron emission) | Research purposes |
| ¹⁴³Eu | 143 | 2.59 days | β⁻ decay | Research purposes |
| ¹⁴⁵Eu | 145 | 5.93 days | β⁻ decay | Research, nuclear physics studies |
| ¹⁴⁶Eu | 146 | 4.61 days | β⁻ decay | Research, nuclear physics studies |
| ¹⁴⁷Eu | 147 | 24.1 days | β⁻ decay | Research, tracer studies |
| ¹⁴⁸Eu | 148 | >1 year | β⁻ decay | Potential research applications |
| ¹⁵⁰Eu | 150 | 36.9 years | β⁻ decay | Used in nuclear science research |
| ¹⁵¹Eu | 151 | Stable | – | Natural abundance, neutron absorber |
| ¹⁵²Eu | 152 | 13.537 years | β⁻ decay | Used as a dopant in nuclear materials |
| ¹⁵³Eu | 153 | Stable | – | Natural abundance, used in nuclear reactors |
| ¹⁵⁴Eu | 154 | 8.593 years | β⁻ decay | Used in medicine and industry |
| ¹⁵⁵Eu | 155 | 4.76 years | β⁻ decay | Research, medical applications |
This article illuminated the remarkable role of Europium in today’s technological and industrial landscapes. From vivid displays and secure currencies to efficient lighting and advanced research, Europium’s unique properties catalyze innovations that touch nearly every aspect of modern life. Its applications underscore the critical importance of rare earth elements in fostering technological advancements and enhancing daily experiences.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Europium?
63
64
65
66
What is the chemical symbol for Europium?
Er
Es
Eu
E
Europium belongs to which group of elements?
Transition metals
Alkali metals
Lanthanides
Actinides
What is the most common oxidation state of Europium in its compounds?
+1
+2
+3
+4
Europium is primarily used in which industry?
Construction
Electronics
Agriculture
Pharmaceuticals
What is the primary application of Europium in consumer electronics?
As a conductor
As a semiconductor
In phosphorescent materials
In batteries
Which isotope of Europium is most abundant?
Europium-151
Europium-152
Europium-153
Europium-154
Europium has notable applications in which type of security features?
Digital signatures
Watermarks
Anti-counterfeiting measures
Encryption keys
What color does Europium emit when used in phosphorescent materials?
Green
Red
Blue
Yellow
Europium is part of which period in the periodic table?
Period 5
Period 6
Period 7
Period 8
Before you leave, take our quick quiz to enhance your learning!

