What is the atomic number of Fluorine?
7
8
9
10
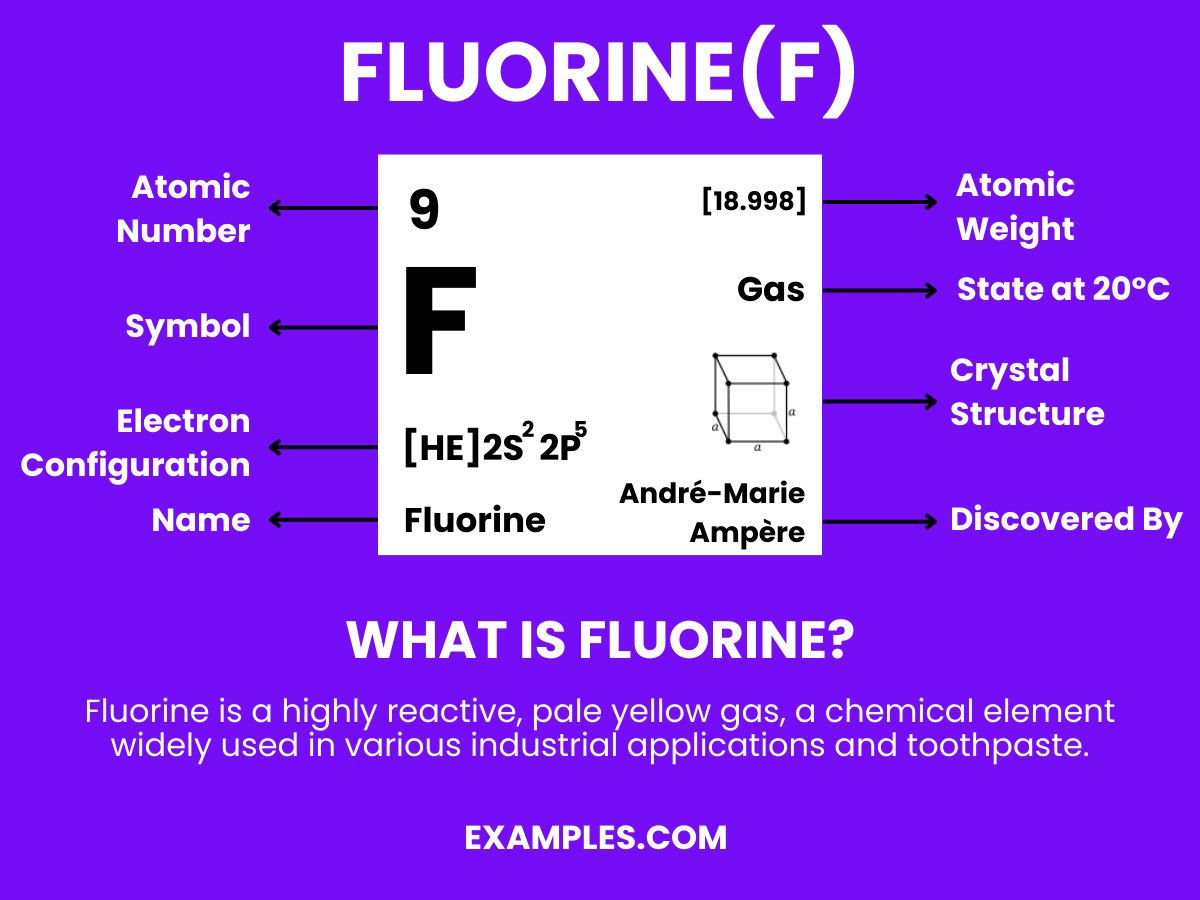
Dive into the dynamic world of Fluorine, an element of mystery and immense scientific value. This guide illuminates the pivotal role Fluorine plays in chemical reactions, especially when it bonds with Hydrogen to form hydrofluoric acid. Teachers and students alike will appreciate the real-world examples, simplifying complex concepts. Understand Fluorine’s reactivity, its presence in everyday life, and its significance in health and industry, all in a language that resonates with educators and learners.
Fluorine is a highly reactive, pale yellow gas and the most electronegative element in the periodic table. It’s known for its incredible ability to react with nearly all elements, including noble gases under certain conditions. In simple terms, Fluorine is an element that forms compounds by stealing electrons from other elements, a process vital to many chemical reactions in industries and organic chemistry. Its reactivity is why it’s used in many everyday products, from toothpaste to pharmaceuticals, making it a fascinating subject for teachers to explore with their students.
| Hydrogen | Sulfur |
| Carbon | Chlorine |
| Nitrogen | Selenium |
| Oxygen | Bromine |
| Phosphorus | Iodine |
Fluorine (F) is a highly reactive, pale yellow gas at room temperature and is the most electronegative and reactive of all the elements. Its unique properties make it a fascinating element in the periodic table. Here are some detailed chemical properties of fluorine:
| Property | Description / Value |
|---|---|
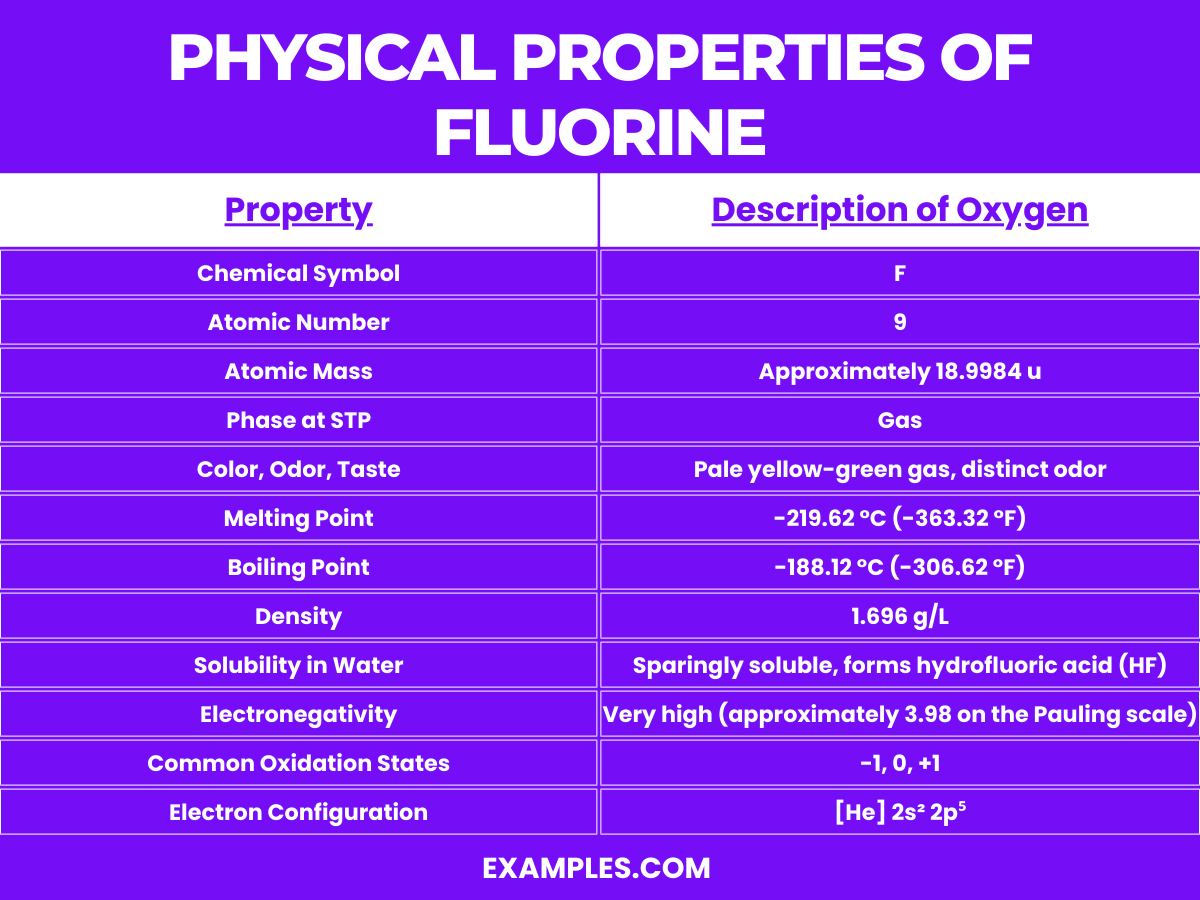
| Melting Point | -219.67°C (-363.41°F) |
| Boiling Point | -188.11°C (-306.60°F) |
| Thermal Conductivity | 0.0277 W/(m·K) |
| Specific Heat | 0.824 J/(g·K) at 298K |
| Heat of Vaporization | 6.51 kJ/mol at boiling point |
| Heat of Fusion | 0.510 kJ/mol at melting point |
| Property | Description / Value |
|---|---|
| Phase at STP | Gas |
| Density | 1.696 g/L at 0°C, 101.325 kPa (gas) |
| Approximately 1.505 g/cm³ at -188°C (liquid) | |
| Solubility in Water | Reacts with water |
| Color | Pale yellow (in its gas and liquid form) |
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Diamagnetic |
| Electrical Conductivity | Non-conductor (Fluorine is a dielectric material) |
| Property | Description / Value |
|---|---|
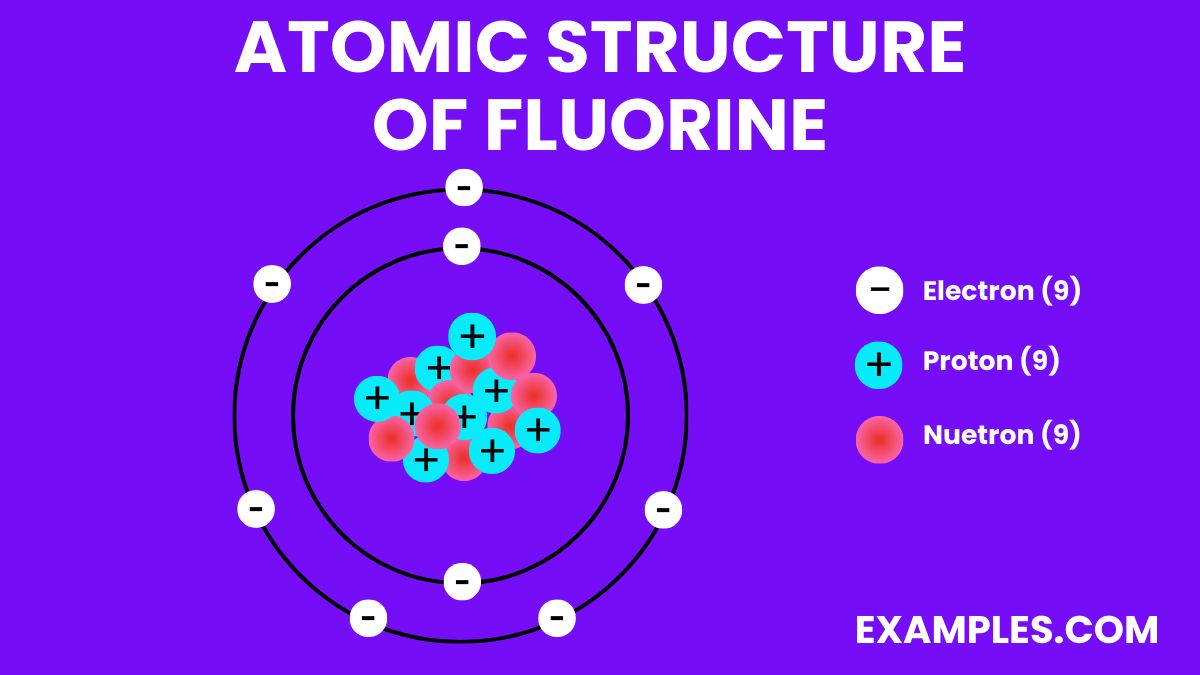
| Atomic Number | 9 |
| Atomic Mass | 18.998403163 u |
| Neutron Cross Section | 0.0096 barns (for ¹⁹F) |
| Isotopes | ¹⁹F is the only stable isotope |
| Radioactivity | Fluorine has several radioactive isotopes, with ^18F being the most notable for its use in PET scans, having a half-life of 109.77 minutes |
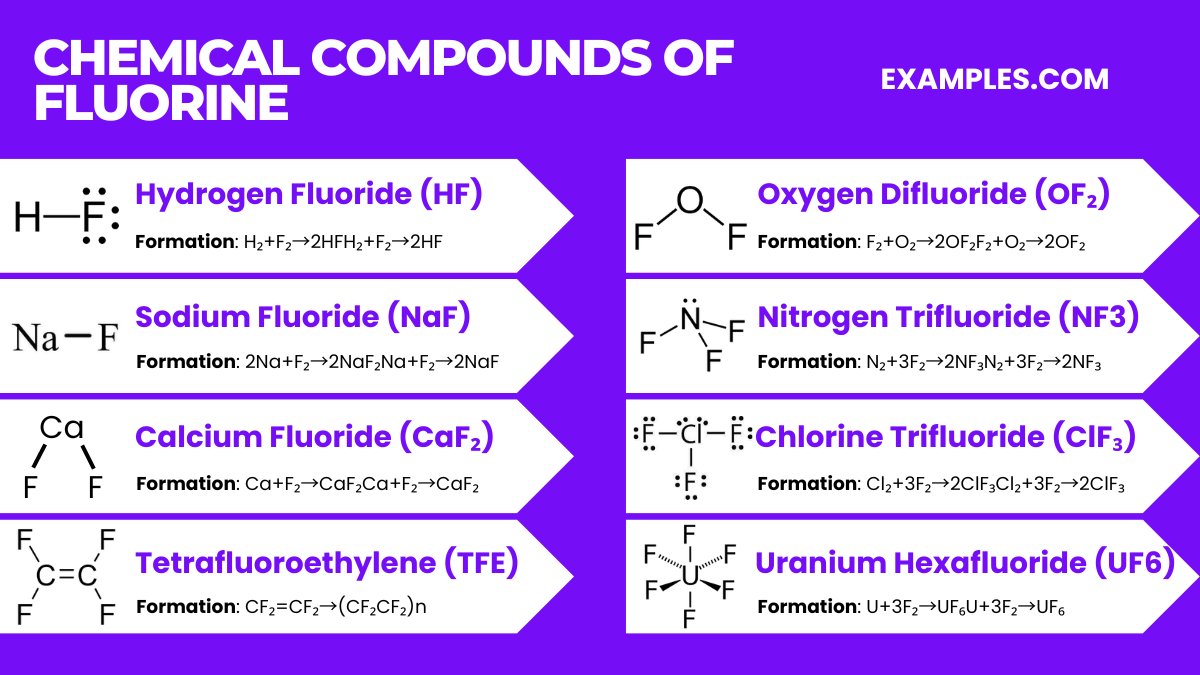
Fluorine forms a wide variety of compounds with almost all elements due to its high reactivity and electronegativity. Here are some of the most notable compounds of fluorine, along with their properties and chemical equations:
Fluorine has several isotopes, but fluorine-19 is the only stable isotope and naturally occurring isotope. Here is a table describing some of the isotopes of fluorine, focusing on fluorine-19 and a few others that have been identified:
| Isotope | Natural Abundance or Mode of Production | Half-Life | Notes |
|---|---|---|---|
| Fluorine-18 | Produced in cyclotrons through the bombardment of oxygen | 109.77 minutes | Used in PET (Positron Emission Tomography) scans in medical imaging. |
| Fluorine-19 | 100% natural abundance | Stable | The only stable and naturally occurring isotope of fluorine. It is used in various chemical applications and studies. |
| Fluorine-20 | Produced synthetically in nuclear reactors or particle accelerators | 11.07 seconds | Used for research purposes, including nuclear physics experiments. |
This table lists some of the isotopes of fluorine, but it should be noted that all isotopes of fluorine other than fluorine-19 are radioactive and have short half-lives, making them less commonly encountered outside of scientific research.
The commercial production of fluorine involves several steps and requires careful control due to the element’s highly reactive and corrosive nature. Here’s a detailed look at the process:
The primary source of fluorine for commercial production is the mineral fluorite (calcium fluoride, CaF₂), which is widely available. The mineral is first converted to hydrogen fluoride (HF) before it is used to produce elemental fluorine.
The health effects of fluorine largely depend on its form (gas or compound) and the concentration or dosage. Here are some of the notable health impacts:
The environmental impact of fluorine and its compounds varies with the form and concentration:
Fluorine is primarily used in toothpaste, pharmaceuticals, Teflon manufacturing, refrigerants, and uranium enrichment processes.
Fluorine can cause severe respiratory issues and skin burns; however, in low doses, it strengthens teeth and bones.
Fluorine is most commonly found in the mineral fluorite (CaF2), used extensively in industries and naturally abundant worldwide.
Fluorine is a highly reactive, pale yellow gas at room temperature, not a metal. It’s the most electronegative and reactive of all the elements.
Fluorine is a highly reactive and versatile element with widespread applications in industry, medicine, and consumer products. While beneficial in controlled amounts, its toxicity and environmental impact necessitate careful handling and regulation. Understanding fluorine’s dual nature is key to harnessing its benefits and minimizing risks, making it a fascinating subject in chemistry and beyond.

Dive into the dynamic world of Fluorine, an element of mystery and immense scientific value. This guide illuminates the pivotal role Fluorine plays in chemical reactions, especially when it bonds with Hydrogen to form hydrofluoric acid. Teachers and students alike will appreciate the real-world examples, simplifying complex concepts. Understand Fluorine’s reactivity, its presence in everyday life, and its significance in health and industry, all in a language that resonates with educators and learners.

Fluorine is a highly reactive, pale yellow gas and the most electronegative element in the periodic table. It’s known for its incredible ability to react with nearly all elements, including noble gases under certain conditions. In simple terms, Fluorine is an element that forms compounds by stealing electrons from other elements, a process vital to many chemical reactions in industries and organic chemistry. Its reactivity is why it’s used in many everyday products, from toothpaste to pharmaceuticals, making it a fascinating subject for teachers to explore with their students.
Formula: F₂
Composition: Consists of two fluorine atoms.
Bond Type: The two fluorine atoms are connected by a strong single bond.
Molecular Structure: Diatomic, meaning it contains two atoms forming an F-F bond.
Electron Configuration: Each fluorine atom has seven valence electrons, with a total of fourteen electrons when in F₂ form.
Significance: Fluorine is a highly reactive element and is the most electronegative of all the elements, making it essential in various chemical processes and applications.
Role in Chemistry: Fluorine’s reactivity allows it to form a wide range of compounds, including fluorides, which are important in dental health and as catalysts in many industrial processes. It is also used in the production of refrigerants, polymers, and pharmaceuticals. Fluorine is often used to enhance the properties of materials due to its unique chemical characteristics.


Fluorine (F) is a highly reactive, pale yellow gas at room temperature and is the most electronegative and reactive of all the elements. Its unique properties make it a fascinating element in the periodic table. Here are some detailed chemical properties of fluorine:
High Electronegativity: Fluorine has the highest electronegativity of all elements, with a Pauling scale value of 3.98. This high electronegativity means fluorine has a strong tendency to attract electrons towards itself, making it extremely reactive.
Reactivity: Fluorine reacts with almost all organic and inorganic substances, including water, glass, and most metals. It forms compounds with most other elements, even with noble gases under certain conditions.
Hydrogen Fluoride (HF): When fluorine reacts with hydrogen, it forms hydrogen fluoride. H₂+F₂→2HFH₂+F₂→2HF
Metal Fluorides: Most metals react vigorously with fluorine to form metal fluorides, such as sodium fluoride (NaF) and calcium fluoride (CaF₂). 2Na+F₂→2NaF₂Na+F₂→2NaF
Fluorine is the strongest oxidizing agent and can oxidize substances that oxygen cannot. It can even oxidize oxygen in some reactions, forming compounds like oxygen difluoride (OF₂).
Example Equation: F₂+O₂→2OF₂F₂+O₂→2OF₂
Fluorides are Generally Stable: Compounds containing fluorine, known as fluorides, are generally very stable due to the strong F-F bond in molecular fluorine and the high lattice energies of ionic fluorides.
Volatility of Fluorides: Some fluorides, like UF₆ (uranium hexafluoride), are volatile, which is crucial in processes like uranium enrichment.
Natural Abundance: Fluorine has only one stable isotope, fluorine-19. Its presence is uniform in all fluorine-containing substances.
Highly Toxic: Fluorine gas is extremely toxic and can cause severe chemical burns. It must be handled with extreme care.
Corrosiveness: Due to its high reactivity, fluorine is highly corrosive and can damage equipment and living tissue.
Fluorocarbons: Fluorine forms a variety of stable organic compounds, such as Teflon and other fluorocarbons, which have unique properties like high thermal stability and hydrophobicity.
Reaction with Hydrocarbons: Fluorine reacts with hydrocarbons to form fluorinated organic compounds, often altering their properties significantly.
Property | Description / Value |
|---|---|
Melting Point | -219.67°C (-363.41°F) |
Boiling Point | -188.11°C (-306.60°F) |
Thermal Conductivity | 0.0277 W/(m·K) |
Specific Heat | 0.824 J/(g·K) at 298K |
Heat of Vaporization | 6.51 kJ/mol at boiling point |
Heat of Fusion | 0.510 kJ/mol at melting point |
Property | Description / Value |
|---|---|
Phase at STP | Gas |
Density | 1.696 g/L at 0°C, 101.325 kPa (gas) |
Approximately 1.505 g/cm³ at -188°C (liquid) | |
Solubility in Water | Reacts with water |
Color | Pale yellow (in its gas and liquid form) |
Property | Description / Value |
|---|---|
Magnetic Susceptibility | Diamagnetic |
Electrical Conductivity | Non-conductor (Fluorine is a dielectric material) |
Property | Description / Value |
|---|---|
Atomic Number | 9 |
Atomic Mass | 18.998403163 u |
Neutron Cross Section | 0.0096 barns (for ¹⁹F) |
Isotopes | ¹⁹F is the only stable isotope |
Radioactivity | Fluorine has several radioactive isotopes, with ^18F being the most notable for its use in PET scans, having a half-life of 109.77 minutes |

Fluorine forms a wide variety of compounds with almost all elements due to its high reactivity and electronegativity. Here are some of the most notable compounds of fluorine, along with their properties and chemical equations:
Properties: Hydrogen fluoride is a colorless gas or liquid that is highly corrosive and toxic. It is used in the production of refrigerants, herbicides, pharmaceuticals, and high-octane gasoline.
Formation Equation: H₂+F₂→2HFH₂+F₂→2HF
Properties: Metal fluorides vary widely in their properties depending on the metal. They are generally very stable and are used in various applications, including as catalysts, in optical coatings, and in nuclear reactors.
Examples and Equations:
Sodium Fluoride (NaF): Used in toothpaste and water fluoridation. 2Na+F₂→2NaF₂Na+F₂→2NaF
Calcium Fluoride (CaF₂): Occurs naturally as the mineral fluorite and is used in the optical industry. Ca+F₂→CaF₂Ca+F₂→CaF₂
Properties: Fluorocarbons, like Teflon (PTFE), are compounds containing carbon and fluorine. They are characterized by their high thermal stability, chemical inertness, and non-stick properties.
Examples and Equations:
Tetrafluoroethylene (TFE), the monomer for Teflon (PTFE): nCF₂=CF₂→(CF₂CF₂)n
Properties: Oxygen fluorides, like OF₂ and O₂F₂, are extremely potent oxidizers and are highly reactive.
Examples and Equations:
Oxygen Difluoride (OF₂): It is used as an oxidizing agent in rocket fuel. F₂+O₂→2OF₂F₂+O₂→2OF₂
Properties: Nitrogen fluorides are a group of compounds that include nitrogen trifluoride (NF3) and are generally used in industrial applications.
Examples and Equations:
Nitrogen Trifluoride (NF3): Used in the cleaning of silicon wafers in the electronics industry. N₂+3F₂→2NF₃N₂+3F₂→2NF₃
Properties: Halogen fluorides are interhalogen compounds formed between fluorine and other halogens. They are highly reactive and often used as fluorinating agents.
Examples and Equations:
Chlorine Trifluoride (ClF₃): Known for its aggressive reactivity and is used in nuclear reactor fuel processing. Cl₂+3F₂→2ClF₃Cl₂+3F₂→2ClF₃
Properties: Uranium hexafluoride is a compound used in the uranium enrichment process. It is volatile and reacts with water, releasing fumes of HF.
Formation Equation: U+3F₂→UF₆U+3F₂→UF₆
Fluorine has several isotopes, but fluorine-19 is the only stable isotope and naturally occurring isotope. Here is a table describing some of the isotopes of fluorine, focusing on fluorine-19 and a few others that have been identified:
Isotope | Natural Abundance or Mode of Production | Half-Life | Notes |
|---|---|---|---|
Fluorine-18 | Produced in cyclotrons through the bombardment of oxygen | 109.77 minutes | Used in PET (Positron Emission Tomography) scans in medical imaging. |
Fluorine-19 | 100% natural abundance | Stable | The only stable and naturally occurring isotope of fluorine. It is used in various chemical applications and studies. |
Fluorine-20 | Produced synthetically in nuclear reactors or particle accelerators | 11.07 seconds | Used for research purposes, including nuclear physics experiments. |
This table lists some of the isotopes of fluorine, but it should be noted that all isotopes of fluorine other than fluorine-19 are radioactive and have short half-lives, making them less commonly encountered outside of scientific research.
Fluorinated Pharmaceuticals: Many pharmaceuticals are fluorinated to enhance their biological activity and stability. Examples include certain antidepressants and anti-cancer drugs.
Dental Care Products: Fluorine compounds, like sodium fluoride, are used in toothpaste and water fluoridation to prevent dental caries.
Uranium Enrichment: Uranium Hexafluoride (UF₆) is used in the gas diffusion process to enrich uranium, crucial for nuclear fuel and weapons.
Chemical Manufacturing: Fluorine is used in the manufacturing of a wide range of chemical products, including Teflon (PTFE), refrigerants, and other fluorocarbons.
Pesticides and Herbicides: Certain fluorine compounds are used as pesticides and herbicides due to their effectiveness in controlling pests and weeds.
Cleaning and Etching: Nitrogen trifluoride and other fluorine compounds are used for cleaning and etching in the production of semiconductors and liquid crystal displays.
Rocket Propellant: Oxygen difluoride and other fluorine compounds are used as oxidizers in rocket propellant systems.
Nuclear Reactor Coolants: Certain fluorine-containing compounds are used as coolants in nuclear reactors due to their stability at high temperatures.
Specialty Plastics and Polymers: The presence of fluorine in polymers like Teflon imparts unique properties such as non-reactivity, high melting points, and reduced friction, making them useful in a wide range of applications from cookware to industrial applications.
The commercial production of fluorine involves several steps and requires careful control due to the element’s highly reactive and corrosive nature. Here’s a detailed look at the process:
The primary source of fluorine for commercial production is the mineral fluorite (calcium fluoride, CaF₂), which is widely available. The mineral is first converted to hydrogen fluoride (HF) before it is used to produce elemental fluorine.
Step 1: Mining and Purification of Fluorite
Fluorite is mined and purified to remove impurities.
Step 2: Acid Treatment
The purified fluorite is then treated with concentrated sulfuric acid.
Equation: CaF₂+H₂SO₄→2HF+CaSO₄CaF₂+H₂SO₄→2HF+CaSO₄
This reaction produces hydrogen fluoride (HF) and a by-product of calcium sulfate (CaSO₄).
Step 1: Preparation of Electrolytic Cells
An electrolytic cell is prepared with an anode and cathode, typically made of carbon or nickel, and the cells are lined with nickel or steel to resist the corrosive effects of fluorine.
Step 2: Electrolysis of Hydrogen Fluoride (HF)
The hydrogen fluoride is mixed with potassium bifluoride (KHF₂) to lower the melting point of the mixture and facilitate electrolysis. This mixture is known as the electrolyte.
Equation: 2HF→H₂+F₂2HF→H₂+F₂
Under the influence of an electric current, hydrogen fluoride dissociates into hydrogen and fluorine at the cathode and anode, respectively.
Step 3: Collection and Purification
The fluorine gas produced at the anode is extremely reactive and corrosive, so it must be collected and purified carefully. Special materials that resist fluorine’s corrosive effects, such as Monel or nickel, are used for handling and storage.
Due to its extreme reactivity and toxicity, the handling of fluorine requires stringent safety protocols, including the use of specialized equipment and facilities designed to contain and control the element.
Continuous monitoring for leaks and personnel safety is mandatory in facilities that produce or use fluorine.
The health effects of fluorine largely depend on its form (gas or compound) and the concentration or dosage. Here are some of the notable health impacts:
Toxicity of Fluorine Gas:
Fluorine gas is highly toxic and can cause severe respiratory damage, eye irritation, and skin burns upon contact.
Hydrogen Fluoride Exposure:
Inhalation or skin contact with hydrogen fluoride (HF) can lead to severe burns, pulmonary edema, and systemic toxicity. HF penetrates tissues deeply and can cause profound damage, including bone erosion.
Fluoride Ion Toxicity:
Ingestion of high levels of fluoride ions can lead to fluorosis, affecting teeth and bones. Dental fluorosis causes discoloration and mottling of teeth, while skeletal fluorosis can result in pain, stiffness, and bone fractures.
Beneficial Effects in Small Amounts:
At low concentrations, fluoride is beneficial for dental health, helping to prevent tooth decay and strengthen bone density.
Threshold of Safety:
The difference between beneficial and harmful doses of fluoride is small, necessitating careful control of fluoride levels in water supplies and toothpaste.
Chronic Exposure:
Long-term exposure to high levels of fluorine compounds can lead to chronic health issues, including bone and joint deformations and neurological problems.
The environmental impact of fluorine and its compounds varies with the form and concentration:
Accumulation in the Environment:
Fluorine compounds can accumulate in the environment, particularly in areas near industrial sites where fluoride is emitted.
Impact on Plants and Animals:
High levels of fluoride can be toxic to plants and animals, causing damage to plant foliage and health issues in animals, including teeth damage and skeletal fluorosis.
Water Contamination:
Excessive fluoride in water sources can lead to environmental fluorosis, affecting entire ecosystems and making water unsafe for consumption by both humans and animals.
Air Pollution:
Industrial emissions of fluorine compounds contribute to air pollution. Fluorine gases and particulates can harm local vegetation and animal life.
Soil Contamination:
Fluoride can accumulate in the soil, affecting soil chemistry and potentially entering the food chain through crops grown in contaminated soil.
Effect on Aquatic Life:
Fluorine compounds in water bodies can affect aquatic life, inhibiting certain enzyme activities and affecting reproduction and growth in fish and other organisms.
Fluorine is primarily used in toothpaste, pharmaceuticals, Teflon manufacturing, refrigerants, and uranium enrichment processes.
Fluorine can cause severe respiratory issues and skin burns; however, in low doses, it strengthens teeth and bones.
Fluorine is most commonly found in the mineral fluorite (CaF2), used extensively in industries and naturally abundant worldwide.
Fluorine is a highly reactive, pale yellow gas at room temperature, not a metal. It’s the most electronegative and reactive of all the elements.
Fluorine is a highly reactive and versatile element with widespread applications in industry, medicine, and consumer products. While beneficial in controlled amounts, its toxicity and environmental impact necessitate careful handling and regulation. Understanding fluorine’s dual nature is key to harnessing its benefits and minimizing risks, making it a fascinating subject in chemistry and beyond.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Fluorine?
7
8
9
10
Fluorine is the most ___________ element in the halogen group.
Electronegative
Electropositive
Stable
Radioactive
Which of the following is NOT a use of fluorine?
In toothpaste to prevent dental cavities
In the manufacture of Teflon
In the production of low-refractive-index glass
In the construction of solar panels
Fluorine gas, at room temperature, appears as:
Colorless
Yellow
Green
Pale yellow
What is the chemical formula for Hydrofluoric acid?
HF
HCl
HBr
HI
Fluorine forms compounds predominantly in the oxidation state of:
-1
0
+1
+2
Which mineral is a common source of fluorine?
Quartz
Calcite
Fluorite
Feldspar
The natural occurrence of elemental fluorine (F2) is rare due to its:
Low reactivity
High reactivity
Stability under normal conditions
Instability under normal conditions
Fluorine's reactivity is primarily due to:
Its large atomic radius
Its small atomic radius
Its high ionization energy
Its low electronegativity
Which industrial process commonly utilizes fluorine?
Steel making
Uranium enrichment
Alcohol distillation
Paper bleaching
Before you leave, take our quick quiz to enhance your learning!

