What is the atomic number of Germanium?
30
32
34
36
Dive into the world of Germanium with our comprehensive guide. This semi-metal element, crucial in electronics and optics, is explained with clarity and depth. Tailored for educators, this guide encompasses its definition, practical applications, and insightful tips. Enhanced with real-world examples, it’s an invaluable resource for teachers to simplify complex concepts. Whether for classroom discussion or curriculum development, understanding Germanium has never been more accessible and engaging.
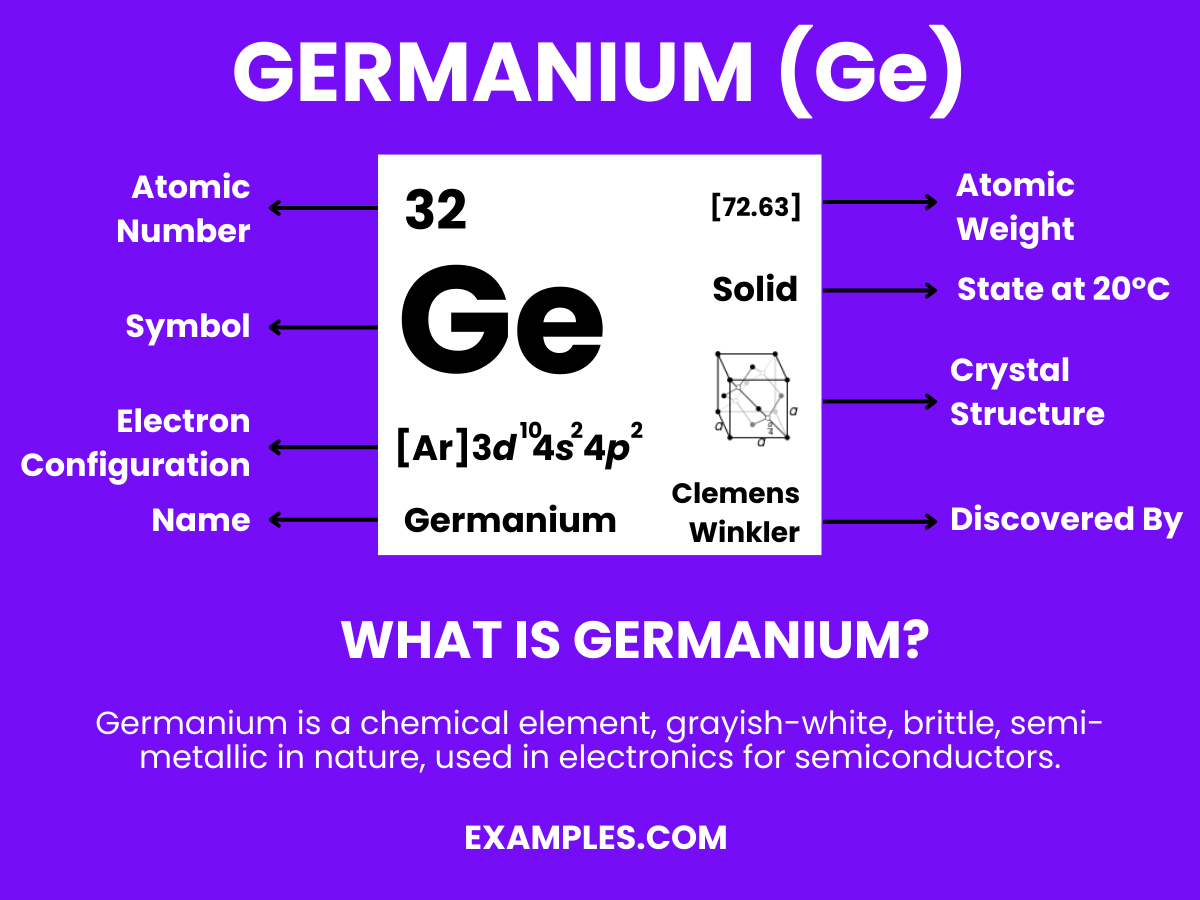
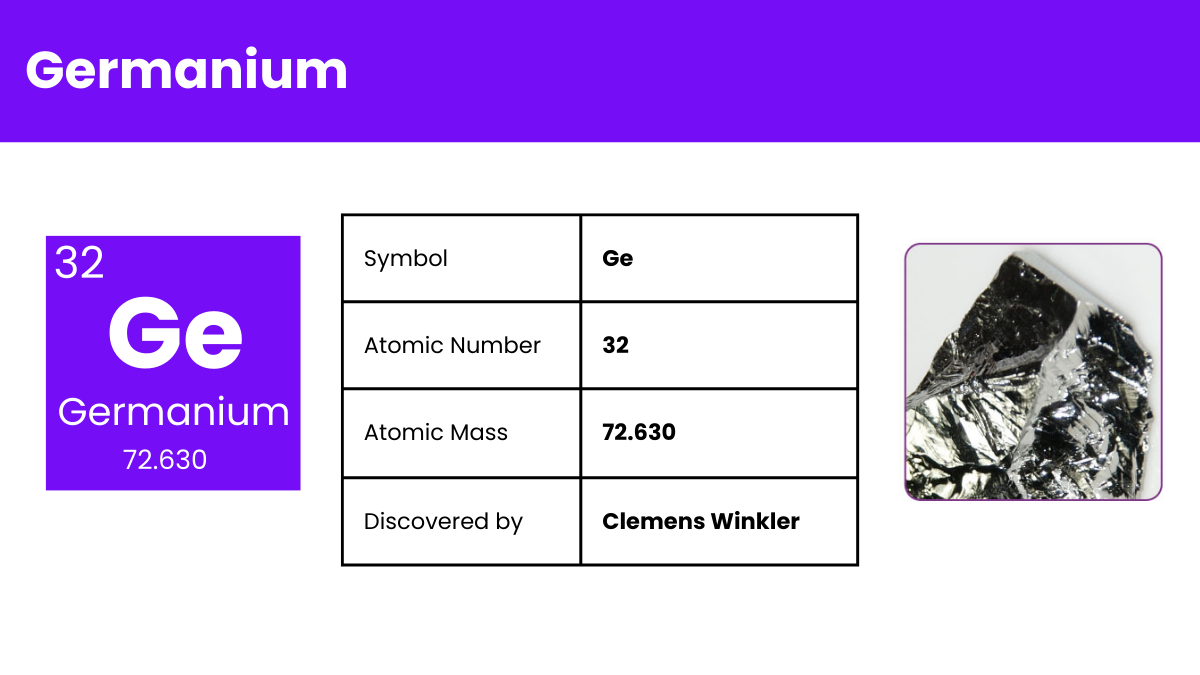
Germanium is a chemical element with the symbol ‘Ge’ and atomic number 32. It’s a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. Germanium is used primarily in semiconductors and fiber optics, known for its ability to conduct electricity in a controlled manner. Its unique properties make it essential in the world of electronics and technology, playing a vital role in advancing modern devices.
| Boron (B) |
| Silicon (Si) |
| Arsenic (As) |
| Antimony (Sb) |
| Tellurium (Te) |
| Property | Description |
|---|---|
| Appearance | Germanium is a lustrous, hard, grayish-white metalloid. |
| Atomic Number | 32 |
| Atomic Weight | 72.630 u |
| Density | 5.323 g/cm³, making it quite dense and heavy for its size. |
| Melting Point | 938.3 °C, indicating its stability at room temperature. |
| Boiling Point | 2833 °C, showcasing its high thermal stability. |
| State at Room Temperature | Solid |
| Crystal Structure | Face-centered cubic, contributing to its semiconducting properties. |
| Conductivity | A semiconductor, with electrical conductivity highly sensitive to impurities. |
| Thermal Conductivity | 59 W/(m·K) at 300 K, indicating moderate heat conduction ability. |
| Band Gap | 0.67 eV at 300 K, important for its use in semiconductors and electronics. |
| Refractive Index | About 4 at wavelengths of 2 μm, significant for optical applications. |
| Hardness | Relatively hard, able to scratch glass and similar materials. |
Germanium is a fascinating element with unique chemical properties:
| Property | Description / Value |
|---|---|
| Melting Point | 938.25°C (1720.85°F) |
| Boiling Point | 2833°C (5131°F) |
| Thermal Conductivity | 60.2 W/(m·K) at 300K |
| Specific Heat | 0.32 J/(g·K) at 25°C |
| Heat of Vaporization | 334 kJ/mol |
| Heat of Fusion | 36.94 kJ/mol |
| Property | Description / Value |
|---|---|
| Phase at STP | Solid |
| Density | 5.323 g/cm³ at 20°C |
| Young’s Modulus | 103 GPa |
| Tensile Strength | 48 MPa |
| Mohs Hardness | ~6 |
| Elastic Modulus | 102.7 GPa |
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Diamagnetic |
| Electrical Conductivity | Semiconductor; varies with doping |
| Band Gap | 0.67 eV at 300K |
| Property | Description / Value |
|---|---|
| Atomic Number | 32 |
| Atomic Mass | 72.630 u |
| Neutron Cross Section | 2.2 barns (for ^74Ge) |
| Isotopes | ^70Ge, ^72Ge, ^73Ge, ^74Ge, ^76Ge |
| Radioactivity | ^76Ge is slightly radioactive with a very long half-life; other isotopes are stable |
Equation: Ge+O₂→GeO₂Ge+O₂→GeO₂
Used in optical materials and as a catalyst.
Equation: Ge+2Cl₂→GeCl₄Ge+2Cl₂→GeCl₄
Essential in the production of optical fiber and semiconductors.
Equation: Ge+2H₂→GeH₄Ge+2H₂→GeH₄
Used in semiconductor industries as a gaseous reducing agent.
Equation: Ge+2S→GeS₂Ge+2S→GeS₂
Important in photonics and as a semiconductor material.
Equation: Ge+2Br₂→GeBr₄Ge+2Br₂→GeBr₄
Used in organic synthesis and chemical research.
Equation: Ge+2I₂→GeI₄Ge+2I₂→GeI₄
Utilized in organic synthesis and chemical vapor deposition.
| Isotope | Half-life | Abundance | Notes |
|---|---|---|---|
| Ge-70 | Stable | 20.84% | Non-radioactive, stable isotope |
| Ge-72 | Stable | 27.54% | Non-radioactive, stable isotope |
| Ge-73 | Stable | 7.73% | Non-radioactive, stable isotope |
| Ge-74 | Stable | 36.28% | Non-radioactive, stable isotope |
| Ge-76 | 1.78 × 10²¹ years | 7.61% | Radioactive, undergoes double beta decay |
| Ge-77 | 11.3 hours | Synthetic | Used in medical and scientific research |
The isotopes of Germanium highlight its stability and varied applications in science and industry.
Germanium is a critical component in the production of fiber optic cables. It is used to increase the refractive index of the core fiber, allowing efficient signal transmission over long distances.
Germanium is transparent to infrared light, making it an essential material in infrared optics. It is widely used in thermal imaging cameras, military night vision devices, and other infrared spectroscopy tools.
Germanium’s semiconducting properties make it valuable in the electronics industry. It is used in transistors, diodes, and as a substrate for the production of various electronic components.
Germanium serves as a substrate material for the production of high-efficiency multijunction photovoltaic cells, which are used in space applications and solar panels.
Certain germanium compounds are used as catalysts in polymerization and other chemical reactions. This application is significant in the production of polyethylene terephthalate (PET) plastics.
The commercial production of germanium involves several key steps:
Germanium, a chemical element found in the environment, is used in various applications, from electronics to dietary supplements. While it has certain beneficial uses, the health effects of germanium can vary significantly based on its form and exposure levels:
In summary, while certain forms of germanium are used in supplements and health products, their safety and efficacy are controversial. The potential health risks, particularly from excessive intake or exposure to inorganic germanium compounds, should be considered seriously.
Germanium’s environmental effects are relatively minimal compared to some other elements, largely due to its low natural abundance and limited mobility in the environment:
Germanium is utilized in fiber optics, infrared optics, solar cell applications, and as a semiconductor in electronics.
Ingested germanium can potentially harm kidneys and liver, though some claim health benefits, which lack scientific backing.
Germanium is found in the Earth’s crust, primarily in sphalerite ore, coal, and certain minerals like argyrodite.
Germanium is not highly radioactive; it is a stable element with only very slight natural radioactivity.
Germanium’s semiconductor properties make it valuable for electronic devices, fiber optics, and as a catalyst in polymerization.
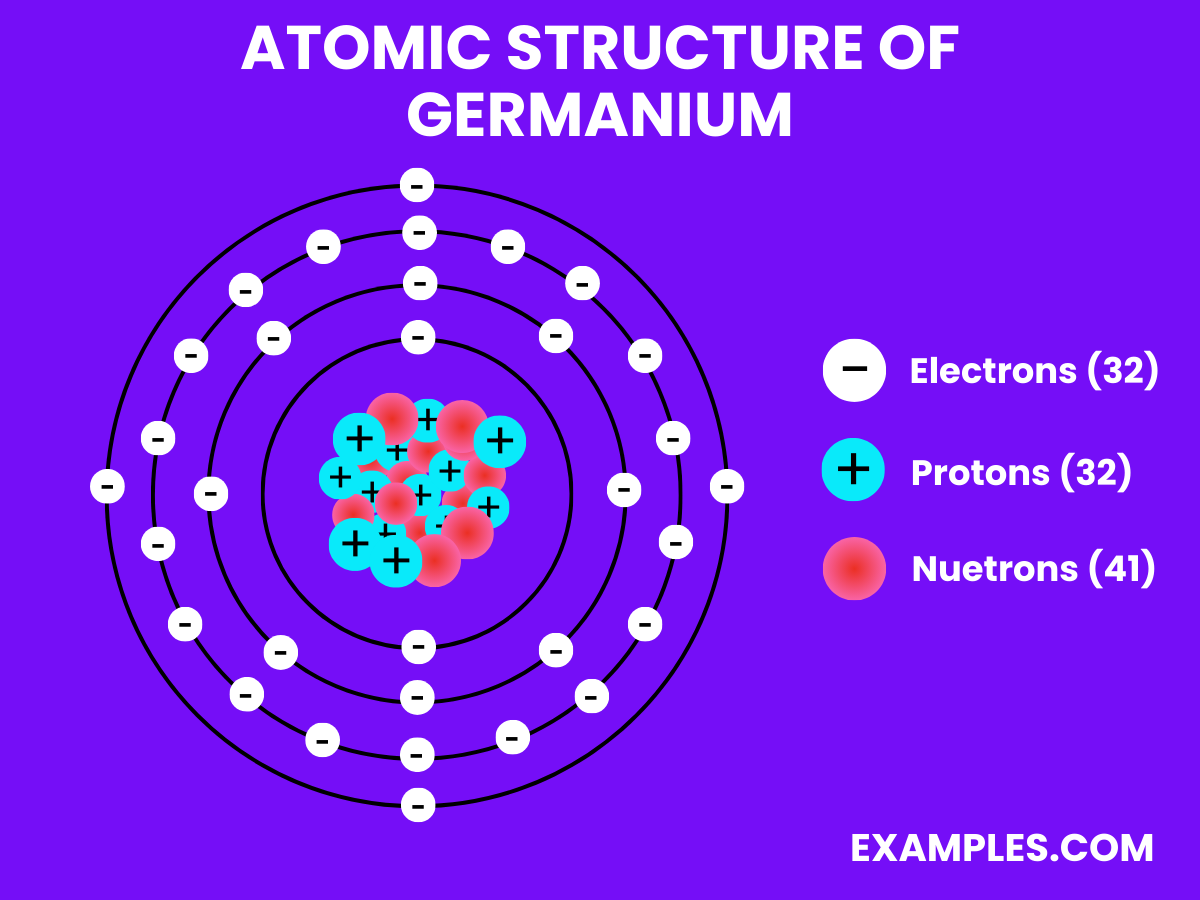
Germanium has 32 electrons, arranged in four electron shells, reflecting its position in Group 14 of the periodic table.
Germanium’s role in semiconductors and fiber optics highlights its significance in communication and technology. Its unique properties offer immense potential for future innovations. Understanding germanium’s applications and handling is essential for harnessing its full capabilities in advancing technological frontiers.

Dive into the world of Germanium with our comprehensive guide. This semi-metal element, crucial in electronics and optics, is explained with clarity and depth. Tailored for educators, this guide encompasses its definition, practical applications, and insightful tips. Enhanced with real-world examples, it’s an invaluable resource for teachers to simplify complex concepts. Whether for classroom discussion or curriculum development, understanding Germanium has never been more accessible and engaging.
Germanium is a chemical element with the symbol ‘Ge’ and atomic number 32. It’s a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. Germanium is used primarily in semiconductors and fiber optics, known for its ability to conduct electricity in a controlled manner. Its unique properties make it essential in the world of electronics and technology, playing a vital role in advancing modern devices.

Formula: Ge
Composition: A single germanium atom.
Bond Type: Germanium forms covalent bonds, characteristic of its four valence electrons.
Molecular Structure: Metallic gray, brittle in its solid form.
Electron Configuration: 32 electrons, with the configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p².
Significance: Used in semiconductors, fiber optics, and infrared optics.
Role in Chemistry: Germanium’s role in semiconductors revolutionized the electronics industry, and it’s vital in organometallic chemistry.


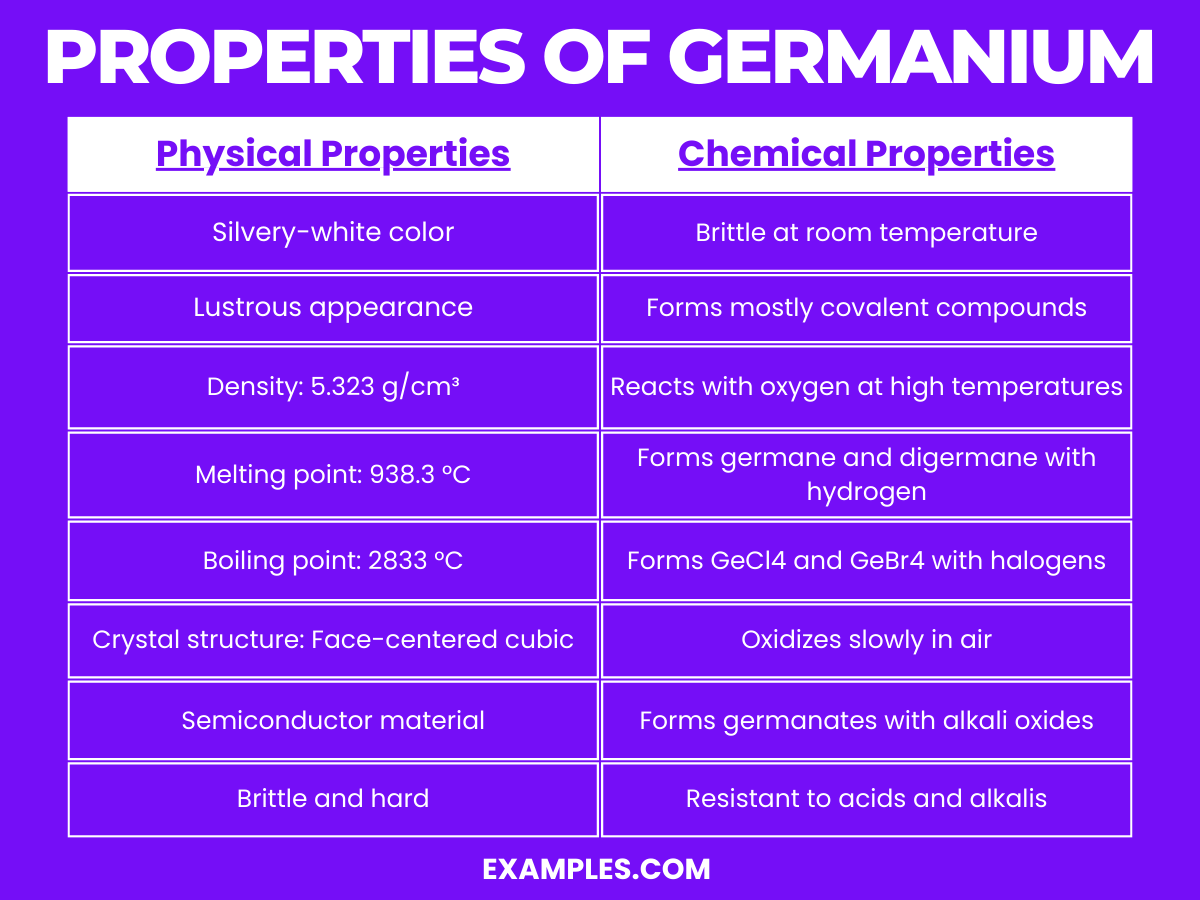
Property | Description |
|---|---|
Appearance | Germanium is a lustrous, hard, grayish-white metalloid. |
Atomic Number | 32 |
Atomic Weight | 72.630 u |
Density | 5.323 g/cm³, making it quite dense and heavy for its size. |
Melting Point | 938.3 °C, indicating its stability at room temperature. |
Boiling Point | 2833 °C, showcasing its high thermal stability. |
State at Room Temperature | Solid |
Crystal Structure | Face-centered cubic, contributing to its semiconducting properties. |
Conductivity | A semiconductor, with electrical conductivity highly sensitive to impurities. |
Thermal Conductivity | 59 W/(m·K) at 300 K, indicating moderate heat conduction ability. |
Band Gap | 0.67 eV at 300 K, important for its use in semiconductors and electronics. |
Refractive Index | About 4 at wavelengths of 2 μm, significant for optical applications. |
Hardness | Relatively hard, able to scratch glass and similar materials. |
Germanium is a fascinating element with unique chemical properties:
Reaction with Oxygen: Germanium reacts with oxygen to form germanium dioxide (GeO₂). The reaction can be represented as: Ge+O₂→GeO₂. This oxide layer protects the metal from further oxidation.
Reaction with Acids and Alkalis: Germanium is resistant to acids but dissolves slowly in hot concentrated sulfuric and nitric acids. It reacts with alkalis to form germanates, Ge+2NaOH+H₂O→Na₂GeO₃+2H₂.
Formation of Germane: When reacting with aqueous alkali, germanium forms germane (GeH₄), a similar compound to methane. The reaction is GeO₂+4LiAlH₄→2GeH₄+2LiAlO₂.
Halogen Reactions: Germanium forms tetrahalides with halogens. For instance, with chlorine, it forms germanium tetrachloride (GeCl4): Ge+2Cl₂→GeCl₄.
Organogermanium Compounds: Germanium forms a variety of organogermanium compounds, similar to organosilicon compounds, used in organometallic chemistry.
Alloy Formation: It readily forms alloys with numerous metals, enhancing their properties for various applications.
Semiconductor Properties: Germanium’s chemical structure allows for controlled doping with other elements, making it an essential material in semiconductor technology.
Property | Description / Value |
|---|---|
Melting Point | 938.25°C (1720.85°F) |
Boiling Point | 2833°C (5131°F) |
Thermal Conductivity | 60.2 W/(m·K) at 300K |
Specific Heat | 0.32 J/(g·K) at 25°C |
Heat of Vaporization | 334 kJ/mol |
Heat of Fusion | 36.94 kJ/mol |
Property | Description / Value |
|---|---|
Phase at STP | Solid |
Density | 5.323 g/cm³ at 20°C |
Young’s Modulus | 103 GPa |
Tensile Strength | 48 MPa |
Mohs Hardness | ~6 |
Elastic Modulus | 102.7 GPa |
Property | Description / Value |
|---|---|
Magnetic Susceptibility | Diamagnetic |
Electrical Conductivity | Semiconductor; varies with doping |
Band Gap | 0.67 eV at 300K |
Property | Description / Value |
|---|---|
Atomic Number | 32 |
Atomic Mass | 72.630 u |
Neutron Cross Section | 2.2 barns (for ^74Ge) |
Isotopes | ^70Ge, ^72Ge, ^73Ge, ^74Ge, ^76Ge |
Radioactivity | ^76Ge is slightly radioactive with a very long half-life; other isotopes are stable |
Equation: Ge+O₂→GeO₂Ge+O₂→GeO₂
Used in optical materials and as a catalyst.
Equation: Ge+2Cl₂→GeCl₄Ge+2Cl₂→GeCl₄
Essential in the production of optical fiber and semiconductors.
Equation: Ge+2H₂→GeH₄Ge+2H₂→GeH₄
Used in semiconductor industries as a gaseous reducing agent.
Equation: Ge+2S→GeS₂Ge+2S→GeS₂
Important in photonics and as a semiconductor material.
Equation: Ge+2Br₂→GeBr₄Ge+2Br₂→GeBr₄
Used in organic synthesis and chemical research.
Equation: Ge+2I₂→GeI₄Ge+2I₂→GeI₄
Utilized in organic synthesis and chemical vapor deposition.
Isotope | Half-life | Abundance | Notes |
|---|---|---|---|
Ge-70 | Stable | 20.84% | Non-radioactive, stable isotope |
Ge-72 | Stable | 27.54% | Non-radioactive, stable isotope |
Ge-73 | Stable | 7.73% | Non-radioactive, stable isotope |
Ge-74 | Stable | 36.28% | Non-radioactive, stable isotope |
Ge-76 | 1.78 × 10²¹ years | 7.61% | Radioactive, undergoes double beta decay |
Ge-77 | 11.3 hours | Synthetic | Used in medical and scientific research |
The isotopes of Germanium highlight its stability and varied applications in science and industry.

Germanium is a critical component in the production of fiber optic cables. It is used to increase the refractive index of the core fiber, allowing efficient signal transmission over long distances.
Germanium is transparent to infrared light, making it an essential material in infrared optics. It is widely used in thermal imaging cameras, military night vision devices, and other infrared spectroscopy tools.
Germanium’s semiconducting properties make it valuable in the electronics industry. It is used in transistors, diodes, and as a substrate for the production of various electronic components.
Germanium serves as a substrate material for the production of high-efficiency multijunction photovoltaic cells, which are used in space applications and solar panels.
Certain germanium compounds are used as catalysts in polymerization and other chemical reactions. This application is significant in the production of polyethylene terephthalate (PET) plastics.
The commercial production of germanium involves several key steps:
Source Material: Germanium is not found in its pure form in nature. It is most commonly extracted from the by-products of zinc ore processing, as well as from certain copper, lead, and silver ores.
Extraction: The extraction process begins with the treatment of these by-products to obtain germanium concentrates. This is typically done through a process called leaching, where the ore is treated with acids or other chemicals to dissolve germanium and separate it from other materials.
Purification: Once germanium is extracted, it undergoes a purification process. One common method is zone refining, where the germanium is heated and slowly passed through a heated zone in a retort. Impurities move to one end of the retort, leaving behind highly pure germanium.
Oxide Reduction: The purified germanium is often in the form of germanium dioxide (GeO₂). To convert this into metallic germanium, a reduction process is used, typically involving a chemical reaction with hydrogen gas at high temperatures.
Final Processing: The resulting germanium metal is further processed to meet specific industry standards. This may involve doping with other elements to enhance its semiconducting properties for electronic applications.
Germanium, a chemical element found in the environment, is used in various applications, from electronics to dietary supplements. While it has certain beneficial uses, the health effects of germanium can vary significantly based on its form and exposure levels:
Organic Germanium Compounds: Some organic germanium compounds are promoted as health supplements, claiming benefits like immune system enhancement and antioxidant properties. However, these claims are not widely supported by scientific evidence. Prolonged intake of these supplements has been linked to potential harmful effects, including kidney damage and other organ dysfunctions.
Inorganic Germanium Compounds: Exposure to inorganic germanium compounds, typically found in industrial settings, can lead to health risks. Inhaling germanium dioxide dust, for instance, can cause lung irritation and, in severe cases, may lead to chronic lung disease. Direct skin contact with germanium compounds can cause irritation.
Germanium as a Trace Element: Germanium is present in trace amounts in the human body, but its biological role is not well understood. There’s no evidence that germanium is essential for human health, and thus, its deficiency doesn’t cause known health issues.
Toxicity: High levels of germanium, particularly from dietary supplements, can be toxic. Symptoms of germanium toxicity include kidney damage, muscle weakness, fatigue, and nerve damage.
In summary, while certain forms of germanium are used in supplements and health products, their safety and efficacy are controversial. The potential health risks, particularly from excessive intake or exposure to inorganic germanium compounds, should be considered seriously.
Germanium’s environmental effects are relatively minimal compared to some other elements, largely due to its low natural abundance and limited mobility in the environment:
Low Abundance: Germanium is not abundant in the Earth’s crust, and it typically occurs in small amounts in certain minerals and ores. Due to this low abundance, its environmental impact is limited.
Industrial Emissions: The primary environmental concern related to germanium is the release of germanium compounds from industrial processes, such as mining and smelting. These emissions can contribute to local soil and water contamination. However, the overall environmental risk is considered low due to germanium’s limited use and release.
Bioaccumulation: There’s limited evidence of germanium bioaccumulating in plants and animals. It doesn’t seem to magnify significantly along the food chain, reducing concerns about its impact on ecosystems and human health through dietary exposure.
Water Solubility: Some germanium compounds are water-soluble, which means they can be transported through water systems. However, their overall environmental mobility is low, and they don’t tend to persist in water bodies.
Recycling and Reuse: Germanium is often recycled, especially from electronic components, reducing its environmental footprint. The recycling process helps limit the need for additional mining and raw material processing.
Germanium is utilized in fiber optics, infrared optics, solar cell applications, and as a semiconductor in electronics.
Ingested germanium can potentially harm kidneys and liver, though some claim health benefits, which lack scientific backing.
Germanium is found in the Earth’s crust, primarily in sphalerite ore, coal, and certain minerals like argyrodite.
Germanium is not highly radioactive; it is a stable element with only very slight natural radioactivity.
Germanium’s semiconductor properties make it valuable for electronic devices, fiber optics, and as a catalyst in polymerization.
Germanium has 32 electrons, arranged in four electron shells, reflecting its position in Group 14 of the periodic table.
Germanium’s role in semiconductors and fiber optics highlights its significance in communication and technology. Its unique properties offer immense potential for future innovations. Understanding germanium’s applications and handling is essential for harnessing its full capabilities in advancing technological frontiers.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Germanium?
30
32
34
36
Germanium is primarily used in which of the following applications?
Glass production
Semiconductor devices
Fuel additives
Food packaging
Which crystal structure does Germanium have at room temperature?
Face-centered cubic
Body-centered cubic
Hexagonal close-packed
Diamond cubic
Which property of Germanium makes it useful in infrared spectroscopes?
Transparency to infrared radiation
Electrical conductivity
Magnetic properties
High melting point
Germanium forms an important alloy with which of the following metals?
Gold
Silver
Aluminum
Lead
What is the common oxidation state of Germanium in most of its compounds?
+1
+2
+3
+4
Exposure to Germanium dust is primarily hazardous to which part of the human body?
Skin
Lungs
Eyes
Kidneys
Which of the following is NOT a source of Germanium?
Coal ash
Zinc ores
Drinking water
Copper ores
The discovery of Germanium was predicted by which scientist?
Dmitri Mendeleev
Marie Curie
Albert Einstein
Isaac Newton
Germanium is recycled primarily from which of the following?
Electronic waste
Plastic waste
Organic waste
Metal scrap
Before you leave, take our quick quiz to enhance your learning!

