Glucose is classified as which type of sugar?
Disaccharide
Monosaccharide
Polysaccharide
Oligosaccharide
Glucose is a simple sugar that is crucial to the energy processes in living organisms, including humans. It’s a type of carbohydrate found naturally in fruits and other foods, and it plays a vital role in providing cells with energy. Chemically, glucose is a covalent compound, meaning it’s made up of atoms sharing electrons, forming strong bonds that hold the molecule together. This simple sugar is essential for many biological functions and is a fundamental topic in the study of chemistry and biology.
Glucose is a type of sugar that is a key source of energy for the cells of our bodies. It comes from the food we eat, especially carbohydrates like bread, fruits, and vegetables. Once inside the body, glucose travels through the bloodstream to our cells, where it is used to produce energy. This energy is vital for our bodies to perform various functions, from running and jumping to thinking and even breathing. Glucose is not just important for humans but for all living organisms. In simple terms, think of glucose as the fuel that powers the engine of life.
| Property | Value |
|---|---|
| Formula | C₆H₁₂O₆ |
| Name | D-(+)-glucose |
| IUPAC Name | 6-(hydroxymethyl)oxane-2, 3, 4, 5-tetrol |
| Alternate Names | dextrose, dextrosol, D-glucose, glucose, grape sugar, sugar, grape |
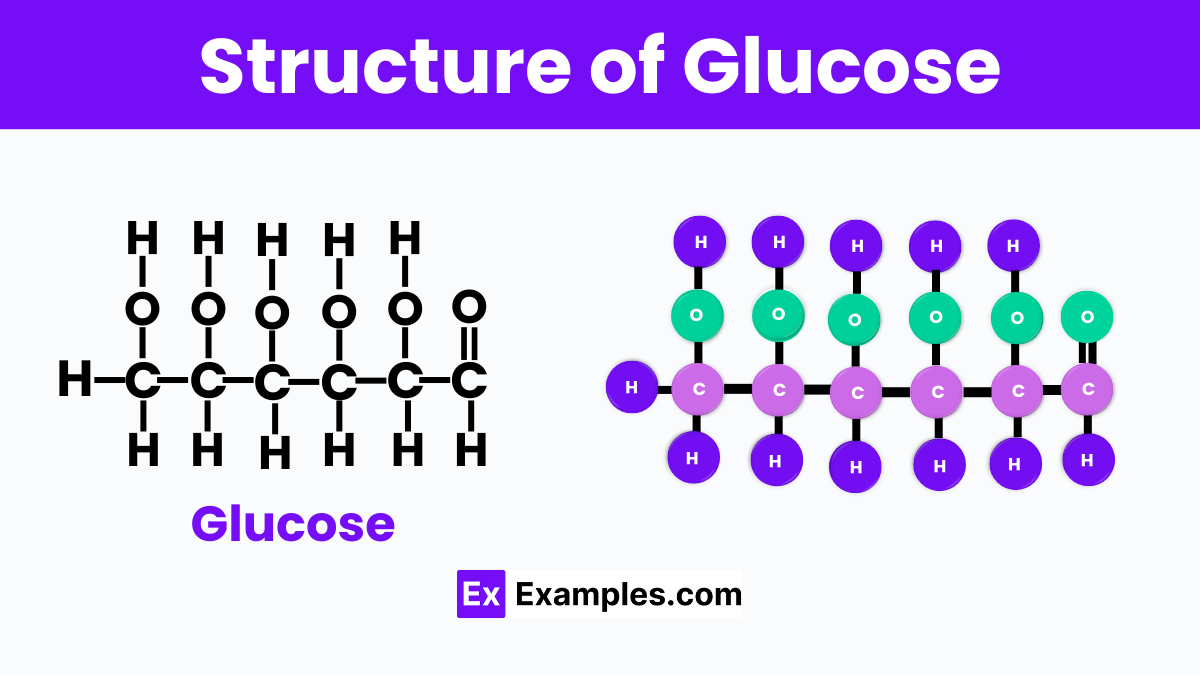
The structure of glucose, which has the chemical formula C₆H₁₂O₆. It is like a tiny building block made up of carbon (C), hydrogen (H), and oxygen (O) atoms. In this structure, six carbon atoms form a chain, with hydrogen and oxygen atoms attached to them in a specific pattern. This arrangement gives glucose its unique properties and enables it to provide energy to living organisms. The atoms in glucose are held together by covalent bonds, which means they share electrons to stay connected. This structure is not just a random assembly; it’s precisely organized to make glucose soluble in water, making it easy for our bodies to transport and use it wherever it’s needed.
The hydrolysis of starch involves breaking down complex carbohydrates into simpler sugars, primarily glucose, by adding water. This reaction can be catalyzed by acids or enzymes. The equation for the acid-catalyzed hydrolysis of starch into glucose is represented as:
In this process, starch (polymer of glucose) is treated with water in the presence of an acid at high temperature. The acid acts as a catalyst to break the glycosidic bonds between the glucose units in starch, resulting in the formation of glucose molecules. This method is widely used in the food industry for the production of glucose syrup from corn or potato starch.
| Property | Description |
|---|---|
| Molecular Weight | 180.16 g/mol |
| Physical State | Solid at room temperature |
| Color | White |
| Taste | Sweet |
| Solubility | Highly soluble in water, sparingly soluble in ethanol, insoluble in ether |
| Melting Point | 146 °C (α-D-glucose) |
| Boiling Point | Decomposes upon heating before boiling |
| Density | 1.54 g/cm³ (solid) |
| Optical Rotation | +52.7° (D-glucose, in water) |
| Specific Rotation | α_D^20 = +52.5 to +53.0° (in water) |
Glucose reacts with alcohols in the presence of an acid catalyst to form glucosides, a type of glycosidic bond.
Equation: C₆H₁₂O₆ + ROH → C₆H₁₁OR + H₂O (R = alkyl group)
Glucose can be oxidized to gluconic acid by mild oxidizing agents like bromine water.
Equation: C₆H₁₂O₆ + Br₂ + H₂O → C₆H₁₂O₇ + 2HBr
It can be reduced to sorbitol (a sugar alcohol) in the presence of hydrogen and a catalyst.
Equation: C₆H₁₂O₆ + H₂ → C₆H₁₄O₆
| Property | Value |
|---|---|
| CAS Registry Number | 50-99-7 |
| Beilstein Number | 1724615 |
| PubChem Compound ID | 206 |
| PubChem Substance ID | 3521 |
| SMILES Identifier | C(C1C(C(C(C(O1)O)O)O)O)O |
| InChI Identifier | InChI=1/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H, 1H2 |
| InChI Key | WQZGKKKJIJFFOK-UHFFFAOYAC |
| EU Number | 200-075-1 |
| RTECS Number | LZ6600000 |
| NSC Number | 406891 |
| Property | Value |
|---|---|
| NFPA Health Rating | 0 |
| NFPA Fire Rating | 0 |
| NFPA Reactivity Rating | 0 |
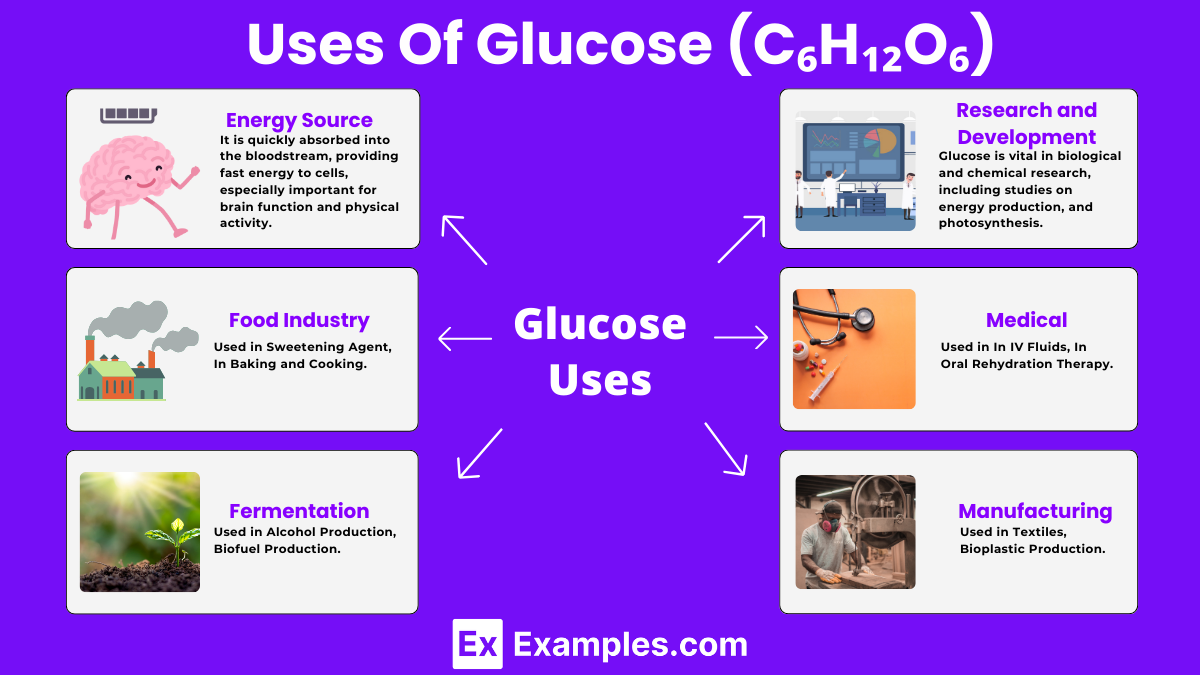
Glucose is the primary fuel for the body’s cells, offering quick energy, particularly crucial for brain function and muscle activity.
It is essential for brain health, aiding cognitive functions like thinking, memory, and learning due to its role as the brain’s main energy source.
Glucose helps in regulating blood sugar levels, with the body’s insulin response facilitating its uptake by the cells, thus providing a steady energy supply.
For athletes and those engaged in high-intensity workouts, glucose is vital for improving performance and endurance by fueling muscle contractions.
Glucose metabolism plays a critical role in maintaining metabolic balance, supporting weight management, and reducing the risk of metabolic syndrome.
It is key in post-exercise recovery, helping to replenish muscle and liver glycogen stores, which speeds up recovery and reduces fatigue.
Glucose provides energy to white blood cells, aiding in the rapid response to infections and promoting healing, thus supporting the immune system.
High glucose, or hyperglycemia, indicates excess sugar in the blood, often signaling diabetes or prediabetes, requiring medical attention.
A normal glucose level ranges between 70-99 mg/dL when fasting and less than 140 mg/dL two hours after eating.
Lower glucose by exercising regularly, eating a balanced diet, managing stress, staying hydrated, and following medical advice.
Glucose is a simple sugar, a building block of carbohydrates. “Sugar” generally refers to table sugar, a disaccharide called sucrose.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Glucose is classified as which type of sugar?
Disaccharide
Monosaccharide
Polysaccharide
Oligosaccharide
In which process is glucose primarily produced in plants?
Respiration
Fermentation
Photosynthesis
Glycolysis
Which enzyme is responsible for converting glucose to glucose-6-phosphate in glycolysis?
Hexokinase
Lactase
Amylase
Pepsin
What is the main storage form of glucose in animals?
Starch
Cellulose
Glycogen
Chitin
Which of the following processes does NOT involve glucose?
Glycolysis
Krebs cycle
Fermentation
Protein synthesis
Which form of glucose is most commonly found in nature?
L-glucose
D-glucose
α-glucose
β-glucose
What is the primary function of glucose in the body?
Structural component
Genetic material
Hormone precursor
Energy source
How is glucose transported into cells?
Facilitated diffusion
Simple diffusion
Active transport
Osmosis
In what form does glucose circulate in the blood?
Free glucose molecules
Glucose-6-phosphate
Glycogen
Glucagon
Which hormone is responsible for lowering blood glucose levels?
Glucagon
Insulin
Epinephrine
Cortisol
Before you leave, take our quick quiz to enhance your learning!

