What is the atomic number of helium?
1
2
3
4
Helium, lighter than air and second only to hydrogen in the universe, is not just for balloons! This comprehensive guide delves into Helium’s fascinating journey from the Big Bang to high-tech industries today. Teachers, equip yourself with engaging examples and understand how Helium floats above the rest in both chemical uniqueness and practical application. Enhance your lessons with the science of Helium, its safe use, and its critical role in research and technology.
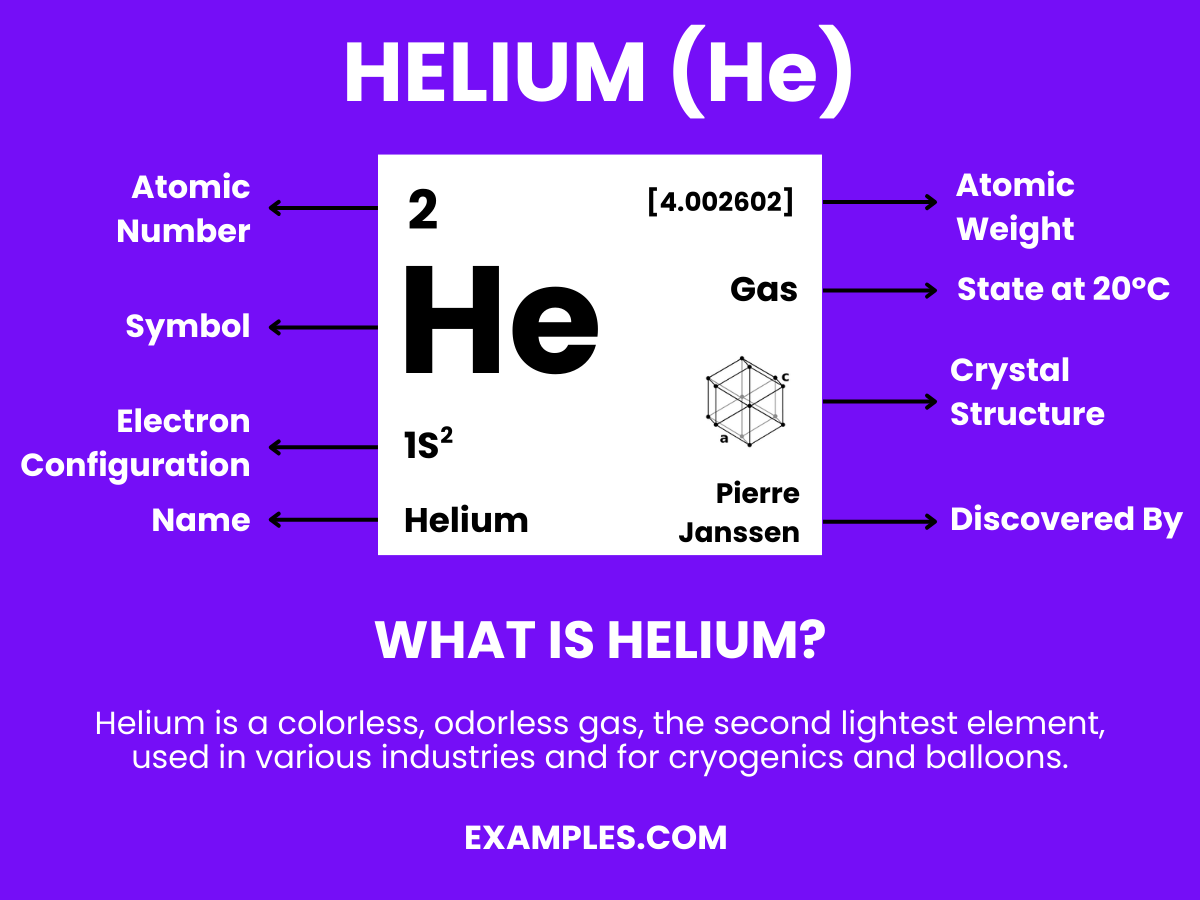
Helium – A noble gas with the chemical symbol He and atomic number 2, Helium is renowned for its inertness and lighter-than-air properties. It’s the second most abundant element in the universe, primarily formed through nuclear fusion in stars. In its earthly applications, Helium is critical in various fields, from scientific research and medical technologies to party balloons and deep-sea diving. Its low boiling point and non-reactivity make it indispensable in cryogenics and as a protective atmosphere for arc welding. Helium is a cornerstone in understanding the universe’s workings and a vital tool in modern technology.
| Neon |
| Argon |
| Krypton |
| Xenon |
| Radon |
| Property | Description |
|---|---|
| Atomic Number | 2 |
| State at Room Temperature | Colorless, odorless, and tasteless gas. |
| Boiling Point | Very low at -268.93°C (-452.07°F), close to absolute zero. |
| Density | Very light, about 0.1786 grams per liter at STP. |
| Solubility | Practically insoluble in water. |
| Speed of Sound | Approximately 3 times faster in helium than in air. |
| Specific Heat | High specific heat, especially at constant volume. |
| Thermal Conductivity | Excellent conductor of heat, superior to all gases except hydrogen. |
Helium’s chemical properties are dominated by its lack of reactivity, which stems from its complete valence electron shell, making it uniquely suitable for a wide variety of applications, especially where non-reactivity is required. Its physical properties, such as low boiling point and high thermal conductivity, make it ideal for cryogenics and other specialized uses.
| Property | Value |
|---|---|
| Atomic Number | 2 |
| Atomic Weight | 4.002602 g/mol |
| Phase at Room Temperature | Gas |
| Density | 0.1786 g/L (at 0°C, 1 atm) |
| Boiling Point | -268.93°C (-452.07°F) |
| Melting Point | -272.20°C (-457.96°F) at 2.5 MPa |
| Specific Heat Capacity (Cp) | 5.193 J/(mol·K) at 25°C |
| Thermal Conductivity | 0.1513 W/(m·K) at 300K |
| Critical Temperature | -267.96°C (-450.33°F) |
| Critical Pressure | 0.22746 MPa |
| Property | Value |
|---|---|
| Electronegativity (Pauling scale) | Essentially 0 (Helium is a noble gas) |
| Ionization Energy | First: 2372.3 kJ/mol, Second: 5250.5 kJ/mol |
| Refractive Index | 1.000035 (at 0°C, 101.325 kPa for λ=589.3 nm) |
| Property | Value |
|---|---|
| Atomic Number | 2 |
| Atomic Weight | 4.002602 g/mol |
| Electron Configuration | 1s² |
| Covalent Radius | 28 pm |
| Van der Waals Radius | 140 pm |
| Ionization Energies | First: 2372.3 kJ/mol, Second: 5250.5 kJ/mol |
| Property | Value |
|---|---|
| Isotopes | Helium-3 (³He), Helium-4 (⁴He) |
| Natural Abundance | ³He: trace, ⁴He: 99.99986% |
| Nuclear Spin | ³He: 1/2, ⁴He: 0 |
| Magnetic Moment | ³He: -2.12762 nuclear magnetons |
| Stable Isotopes | ⁴He |
| Radioactive Isotopes | ³He (stable in terrestrial conditions but considered a primordial nuclide) |
Helium is unique among the elements due to its extreme chemical inertness. As a noble gas with a completely filled valence electron shell, it doesn’t naturally form chemical compounds under standard conditions. However, under extreme conditions, such as very high pressures or in the presence of plasma, it can form exotic compounds. Here are six noteworthy compounds involving helium, although it’s important to note that these are not typically encountered in everyday chemistry:
| Isotope | Atomic Mass | Natural Abundance | Stability | Description |
|---|---|---|---|---|
| Helium-3 (³He) | 3 | Very Rare (~0.0001%) | Stable | Less abundant isotope with one neutron. Used in cryogenics, as a neutron detector, and potential fuel for nuclear fusion. |
| Helium-4 (⁴He) | 4 | Abundant (~99.9999%) | Stable | Most common isotope with two neutrons. Formed in alpha decay of heavier elements and used in cryogenics, as a lifting gas, and in gas chromatography. |
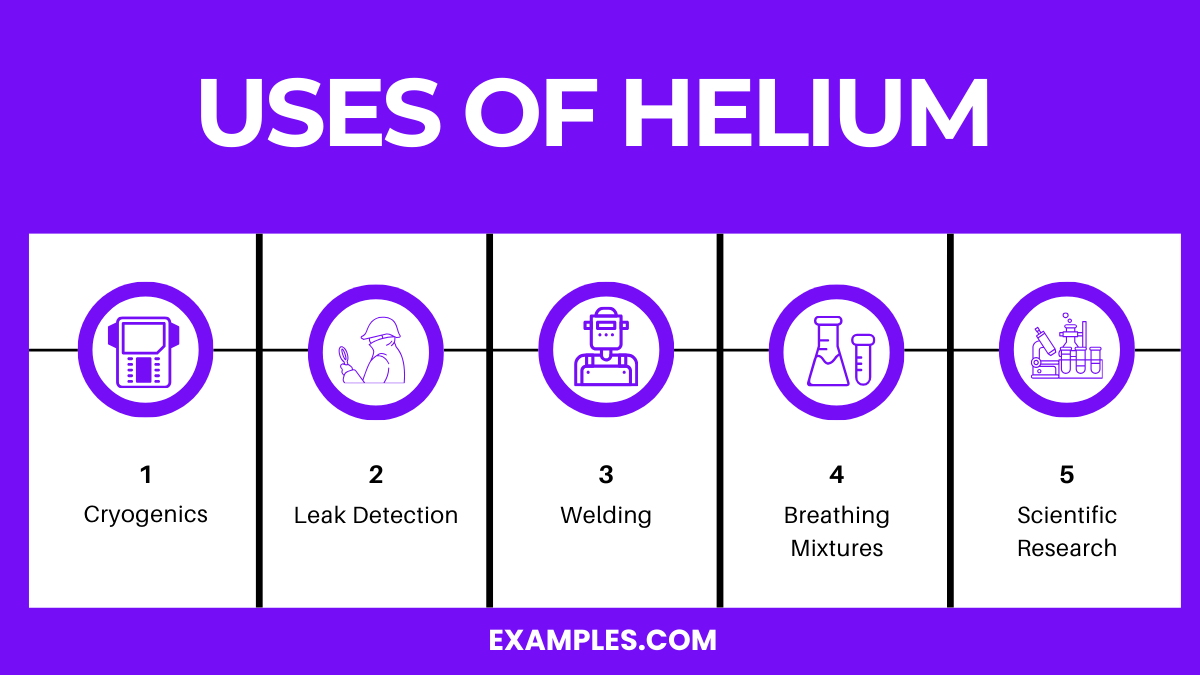
Helium is primarily used in cryogenics, as a lifting gas, in gas chromatography, for leak detection, in arc welding, and in deep-sea diving mixtures.
Yes, helium is a finite resource with increasing concerns about its depletion due to limited reserves and rising demand in various industries.
Helium is formed through natural processes like radioactive decay and nuclear fusion in stars, not by chemical synthesis, making artificial production impractical.
Yes, helium is used as a gas in many applications due to its inertness, including as a protective atmosphere, in balloons, and in scientific research.
Inhaling helium temporarily changes your voice pitch; however, it can be dangerous, causing dizziness, asphyxiation, and in severe cases, death due to oxygen displacement.
Helium is extracted from natural gas reserves where it accumulates over millions of years from radioactive decay of uranium and thorium.
The purpose of helium is diverse: from enabling MRI machines in medicine, lifting balloons, protecting welds, to conducting scientific research due to its unique properties.
Helium serves as an invaluable resource across various fields due to its unique properties. Understanding how to utilize helium effectively is crucial in industries ranging from healthcare to aerospace. As reserves dwindle, appreciating its uses, conserving it, and seeking alternatives become increasingly important. This guide aims to equip you with knowledge and tips to navigate the world of helium efficiently.

Helium, lighter than air and second only to hydrogen in the universe, is not just for balloons! This comprehensive guide delves into Helium’s fascinating journey from the Big Bang to high-tech industries today. Teachers, equip yourself with engaging examples and understand how Helium floats above the rest in both chemical uniqueness and practical application. Enhance your lessons with the science of Helium, its safe use, and its critical role in research and technology.
Helium – A noble gas with the chemical symbol He and atomic number 2, Helium is renowned for its inertness and lighter-than-air properties. It’s the second most abundant element in the universe, primarily formed through nuclear fusion in stars. In its earthly applications, Helium is critical in various fields, from scientific research and medical technologies to party balloons and deep-sea diving. Its low boiling point and non-reactivity make it indispensable in cryogenics and as a protective atmosphere for arc welding. Helium is a cornerstone in understanding the universe’s workings and a vital tool in modern technology.
Formula: He
Composition: A single atom of helium.
Bond Type: Helium atoms do not typically form bonds due to their full valence shell.
Molecular Structure: Monatomic gas.
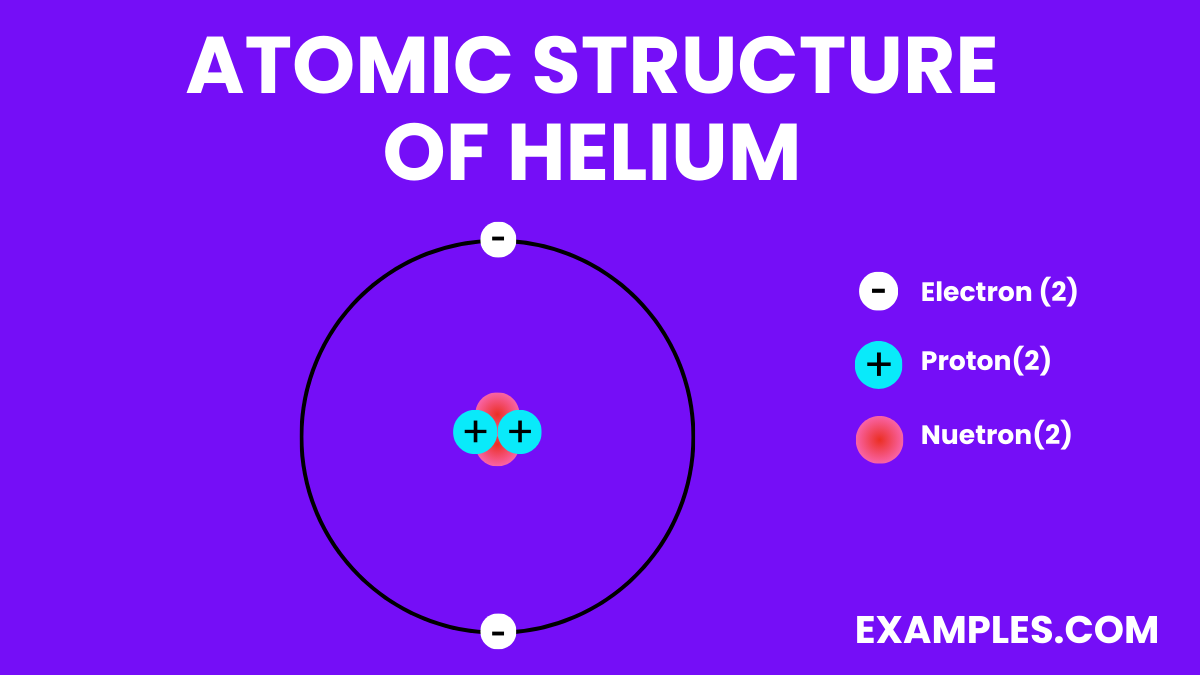
Electron Configuration: Two electrons in the outermost shell, making it highly stable and inert.
Significance: Essential in cryogenics and as a cooling medium, helium is also used in balloons and as an inert gas shield in welding.
Role in Chemistry: Used as a non-reactive carrier gas in gas chromatography and as a protective atmosphere in various applications due to its inertness.


Property | Description |
|---|---|
Atomic Number | 2 |
State at Room Temperature | Colorless, odorless, and tasteless gas. |
Boiling Point | Very low at -268.93°C (-452.07°F), close to absolute zero. |
Density | Very light, about 0.1786 grams per liter at STP. |
Solubility | Practically insoluble in water. |
Speed of Sound | Approximately 3 times faster in helium than in air. |
Specific Heat | High specific heat, especially at constant volume. |
Thermal Conductivity | Excellent conductor of heat, superior to all gases except hydrogen. |
Inertness: Helium is extremely inert and does not react with other elements or compounds under normal conditions. This is due to its filled valence shell (1s²).
No known stable compounds at standard conditions.
Non-flammability: Helium does not burn nor support combustion, making it safe for use in various applications including as a protective atmosphere.
Atomic Size and Electron Configuration: Helium has the smallest atomic radius of all elements because its nucleus is tightly bound, and it has only two electrons.
Electron Configuration: 1s2
Ionization Energy: Helium has the highest first ionization energy of all the elements because removing an electron disrupts its stable, filled electron shell.
First Ionization Energy: 24.5874 eV24.5874eV
Reactivity and Formation of Compounds: Due to its complete electron shell, Helium typically does not form compounds. Some high-pressure and exotic compounds have been observed, but these are not stable under normal conditions.
Examples include molecules like HeNe or ions in extreme conditions but none stable at room temperature or natural environments.
Occurrence in Nature: While Helium is the second most abundant element in the universe, primarily formed through nuclear fusion in stars, it is relatively rare on Earth and is usually extracted from natural gas reserves.
In the universe: HeHe is mostly produced in stars through the proton-proton chain reaction and the carbon-nitrogen-oxygen cycle.
Helium’s chemical properties are dominated by its lack of reactivity, which stems from its complete valence electron shell, making it uniquely suitable for a wide variety of applications, especially where non-reactivity is required. Its physical properties, such as low boiling point and high thermal conductivity, make it ideal for cryogenics and other specialized uses.
Property | Value |
|---|---|
Atomic Number | 2 |
Atomic Weight | 4.002602 g/mol |
Phase at Room Temperature | Gas |
Density | 0.1786 g/L (at 0°C, 1 atm) |
Boiling Point | -268.93°C (-452.07°F) |
Melting Point | -272.20°C (-457.96°F) at 2.5 MPa |
Specific Heat Capacity (Cp) | 5.193 J/(mol·K) at 25°C |
Thermal Conductivity | 0.1513 W/(m·K) at 300K |
Critical Temperature | -267.96°C (-450.33°F) |
Critical Pressure | 0.22746 MPa |
Property | Value |
|---|---|
Electronegativity (Pauling scale) | Essentially 0 (Helium is a noble gas) |
Ionization Energy | First: 2372.3 kJ/mol, Second: 5250.5 kJ/mol |
Refractive Index | 1.000035 (at 0°C, 101.325 kPa for λ=589.3 nm) |
Property | Value |
|---|---|
Atomic Number | 2 |
Atomic Weight | 4.002602 g/mol |
Electron Configuration | 1s² |
Covalent Radius | 28 pm |
Van der Waals Radius | 140 pm |
Ionization Energies | First: 2372.3 kJ/mol, Second: 5250.5 kJ/mol |
Property | Value |
|---|---|
Isotopes | Helium-3 (³He), Helium-4 (⁴He) |
Natural Abundance | ³He: trace, ⁴He: 99.99986% |
Nuclear Spin | ³He: 1/2, ⁴He: 0 |
Magnetic Moment | ³He: -2.12762 nuclear magnetons |
Stable Isotopes | ⁴He |
Radioactive Isotopes | ³He (stable in terrestrial conditions but considered a primordial nuclide) |

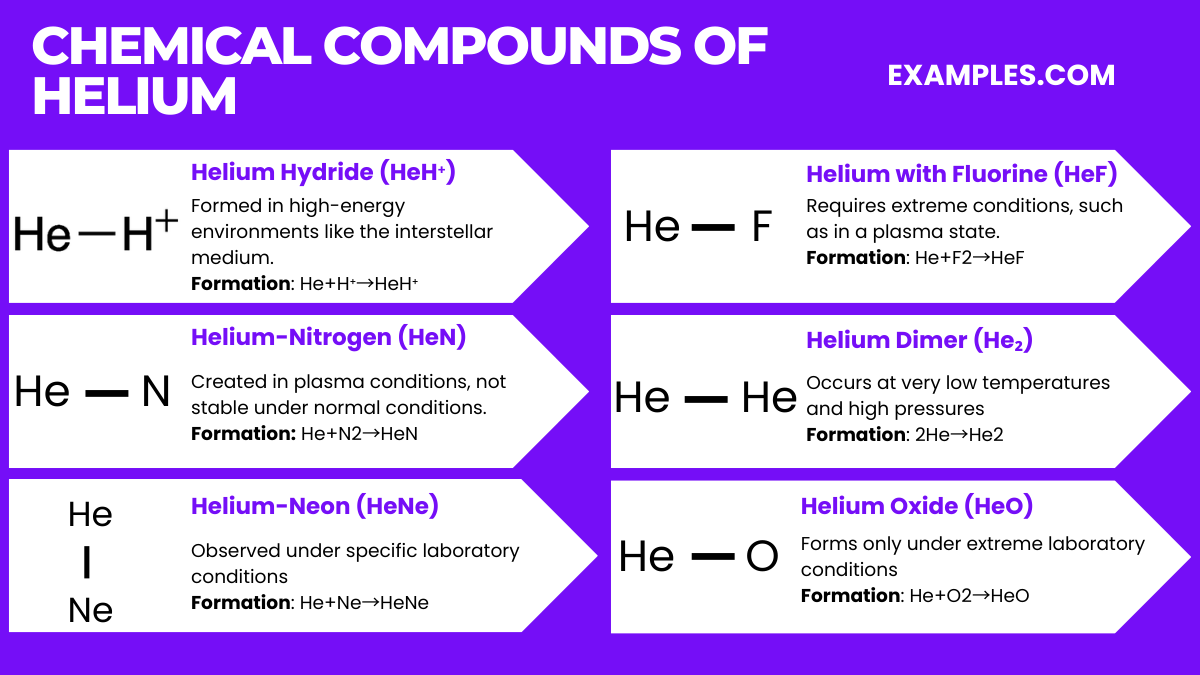
Helium is unique among the elements due to its extreme chemical inertness. As a noble gas with a completely filled valence electron shell, it doesn’t naturally form chemical compounds under standard conditions. However, under extreme conditions, such as very high pressures or in the presence of plasma, it can form exotic compounds. Here are six noteworthy compounds involving helium, although it’s important to note that these are not typically encountered in everyday chemistry:
Helium Hydride (HeH⁺)
Equation: He+H⁺→HeH⁺
Conditions: Formed in high-energy environments like the interstellar medium.
Helium-Nitrogen Compound (HeN)
Equation: He+N₂→HeN
Conditions: Created in plasma conditions, not stable under normal conditions.
Helium-Neon Compound (HeNe)
Equation: He+Ne→HeNe
Conditions: Observed under specific laboratory conditions, primarily in laser technology.
Helium Compound with Fluorine (HeF)
Equation: He+F₂→HeF
Conditions: Requires extreme conditions, such as in a plasma state.
Helium Dimer (He₂)
Equation: 2He→He₂
Conditions: Occurs at very low temperatures and high pressures, often considered a van der Waals molecule.
Helium Oxide (HeO)
Equation: He+O₂→HeO
Conditions: Forms only under extreme laboratory conditions, not a stable compound in normal environments.
Isotope | Atomic Mass | Natural Abundance | Stability | Description |
|---|---|---|---|---|
Helium-3 (³He) | 3 | Very Rare (~0.0001%) | Stable | Less abundant isotope with one neutron. Used in cryogenics, as a neutron detector, and potential fuel for nuclear fusion. |
Helium-4 (⁴He) | 4 | Abundant (~99.9999%) | Stable | Most common isotope with two neutrons. Formed in alpha decay of heavier elements and used in cryogenics, as a lifting gas, and in gas chromatography. |

Cryogenics: Due to its low boiling point, helium is used as a coolant in cryogenics, particularly for cooling superconducting magnets in MRI machines and other scientific equipment.
Lifting Gas: Helium is lighter than air and non-flammable, making it the safer choice over hydrogen for inflating balloons, airships, and blimps.
Gas Chromatography: As an inert gas, helium is used as a carrier in gas chromatography, ensuring substances are efficiently and safely separated and analyzed.
Pressurizing and Purging: In space technology and high-pressure systems, helium is used for pressurizing fuel tanks and purging systems, thanks to its inertness and low density.
Welding: Helium is used as a shielding gas in arc welding, protecting the weld area from atmospheric gases that might corrupt the weld.
Breathing Mixtures: For deep-sea diving, helium is mixed with oxygen to create a breathing gas that avoids the narcotic effect of nitrogen at high pressures (Trimix).
Leak Detection: Due to its small atomic size and inertness, helium is used in detecting leaks in high-vacuum equipment and high-pressure containers.
Scientific Research: Helium’s inertness and extreme conditions (low boiling point, high speed of sound, low solubility) make it valuable for various scientific research applications.
Natural Gas Extraction: The primary commercial source of helium is natural gas, which can contain up to 7% helium depending on the source. Plants cool the natural gas to a low temperature, at which point helium and other gases liquefy and can be separated.
Air Distillation: Though not as common due to its low concentration in the atmosphere, helium can technically be obtained by liquefying and distilling air. This process is not commercially viable due to the high energy cost and low yield.
Radioactive Decay: While not a commercial method, it’s worth noting that helium is also produced through the alpha decay of heavy radioactive elements where an alpha particle (helium nucleus) is emitted.
Refining and Storage: Once extracted, helium is purified to remove any remaining nitrogen, methane, hydrogen, and other impurities. It’s then either liquefied for transport
Inhalation Risks: Inhaling helium directly from a pressurized container can be dangerous, leading to dizziness, light-headedness, or even asphyxiation due to displacement of oxygen in the lungs.
Asphyxiation Hazard: Since helium is lighter than air, it can accumulate in the upper parts of enclosed spaces, displacing oxygen and posing a risk of asphyxiation when inhaled in large quantities.
Effect on Voice: Breathing in helium temporarily changes the sound of a person’s voice, making it much higher. This is due to the speed of sound being faster in helium than in air, but it should be noted that this practice is risky and can lead to serious health effects.
Non-Toxic Nature: Helium is non-toxic and chemically inert, meaning it doesn’t react with other elements or compounds within the human body. It poses no chronic health risks when handled properly.
Minimal Biological Role: Helium has no known biological role in humans or animals, and it’s not absorbed by the body. Any inhaled helium is exhaled and does not accumulate in the body.
Low Reactivity: Being a noble gas, helium is chemically inert and doesn’t react with the environment. It doesn’t contribute to chemical smog, acid rain, or greenhouse gas effects.
Non-renewable Resource: Helium is a finite, non-renewable resource primarily extracted from natural gas. Excessive consumption and wastage can deplete available reserves critical for scientific and medical instruments.
Impact on Wildlife: There are no direct adverse effects of helium on plants or animals due to its inertness. However, the release of balloons filled with helium can lead to litter and pose ingestion and entanglement risks to wildlife.
Atmospheric Escape: Once released into the atmosphere, helium eventually escapes into space due to its light nature. This loss is permanent and contributes to the depletion of earthly helium resources.
Minimal Greenhouse Effect: Helium doesn’t contribute to the greenhouse effect or global warming. Its environmental footprint is mostly related to the energy used and emissions from its extraction and purification processes.
Helium is primarily used in cryogenics, as a lifting gas, in gas chromatography, for leak detection, in arc welding, and in deep-sea diving mixtures.
Yes, helium is a finite resource with increasing concerns about its depletion due to limited reserves and rising demand in various industries.
Helium is formed through natural processes like radioactive decay and nuclear fusion in stars, not by chemical synthesis, making artificial production impractical.
Yes, helium is used as a gas in many applications due to its inertness, including as a protective atmosphere, in balloons, and in scientific research.
Inhaling helium temporarily changes your voice pitch; however, it can be dangerous, causing dizziness, asphyxiation, and in severe cases, death due to oxygen displacement.
Helium is extracted from natural gas reserves where it accumulates over millions of years from radioactive decay of uranium and thorium.
The purpose of helium is diverse: from enabling MRI machines in medicine, lifting balloons, protecting welds, to conducting scientific research due to its unique properties.
Helium serves as an invaluable resource across various fields due to its unique properties. Understanding how to utilize helium effectively is crucial in industries ranging from healthcare to aerospace. As reserves dwindle, appreciating its uses, conserving it, and seeking alternatives become increasingly important. This guide aims to equip you with knowledge and tips to navigate the world of helium efficiently.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of helium?
1
2
3
4
Which group of the periodic table does helium belong to?
Alkali metals
Halogens
Noble gases
Transition metals
Helium is the second most abundant element in the universe. Which is the most abundant?
Oxygen
Hydrogen
Carbon
Nitrogen
What is the primary use of helium in everyday applications?
Making fertilizers
Filling balloons
Fuel for cars
Cooling computers
Helium is a gas at room temperature. What state does helium become when cooled below -269°C?
Liquid
Solid
Plasma
Gas
What is the chemical symbol for helium?
H
Hm
He
Hl
Helium is often used in medical applications. Which of the following is a common use of helium in healthcare?
Anesthetic gas
MRI cooling
Dental fillings
X-ray machines
Helium is the lightest noble gas. What property does helium have that makes it suitable for high-altitude balloons?
It is highly reactive
It is flammable
It is less dense than air
It is heavier than air
Helium is not flammable. Which of the following gases is often compared to helium but is highly flammable?
Nitrogen
Hydrogen
Oxygen
Argon
In what form does helium naturally exist in the Earth's atmosphere?
Solid
Liquid
Gas
Plasma
Before you leave, take our quick quiz to enhance your learning!

