Hemocyanin is primarily found in which organisms?
Vertebrates
Mollusks and arthropods
Plants
Fungi

Hemocyanin is a fascinating protein found in the blood of many mollusks and some arthropods, like lobsters and crabs. Unlike the hemoglobin in human blood that carries oxygen and contains iron. Hemocyanin includes copper, giving the blood a blue color when oxygen binds to it. This protein plays a crucial role in transporting oxygen through the blood, helping these creatures thrive in their environments. The study of such complex compounds enriches our understanding of biological chemistry and highlights the diversity of life’s adaptations to various ecological niches.
Hemocyanin is a copper-containing protein that serves as an oxygen carrier in the circulatory system of many mollusks and arthropods, including octopuses and spiders. Its primary function is to transport oxygen from the respiratory organs to other parts of the body, which is vital for cellular metabolism. Chemically, hemocyanin is not represented by a single molecular formula due to its complex structure consisting of multiple protein subunits and copper ions. The presence of copper, which binds oxygen, causes the blood to turn blue when oxygenated, distinguishing it from the iron based hemoglobin in vertebrates. This compound exemplifies the diverse strategies evolved by organisms to adapt to their environments.
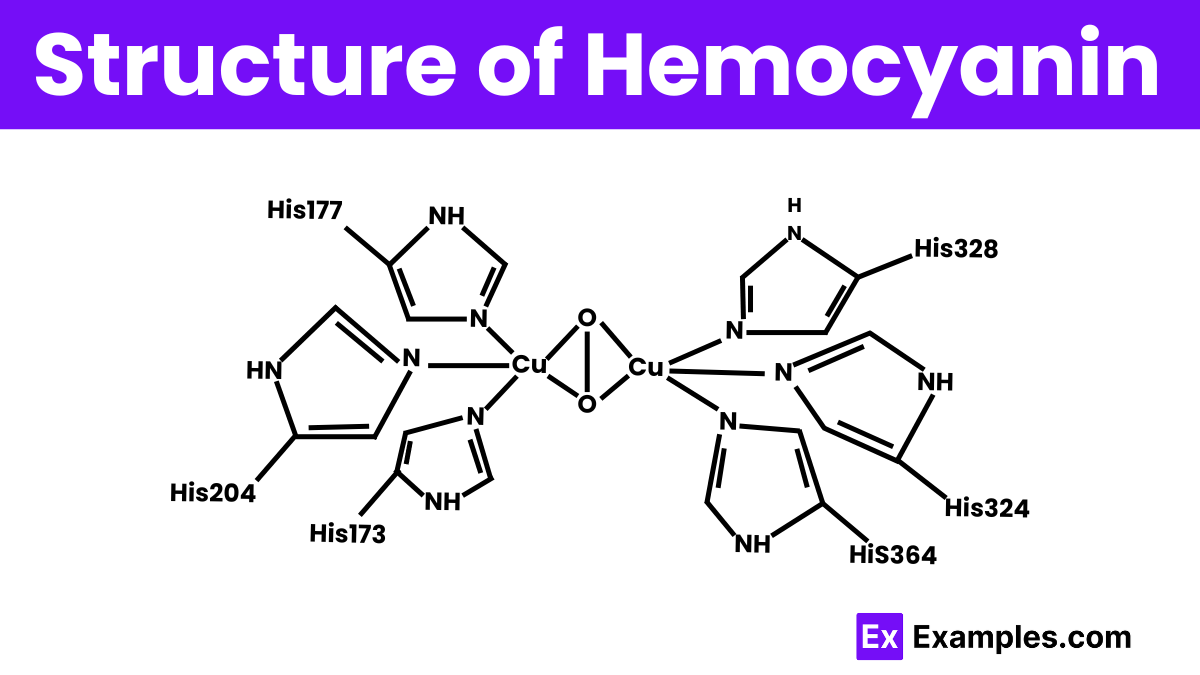
Hemocyanin is a large, multi-subunit protein complex primarily found in the blood of some mollusks and arthropods. It consists of several globular protein subunits, each containing two copper atoms at their active sites. These copper atoms are crucial as they bind oxygen molecules directly, which is essential for transporting oxygen throughout the organism. When oxygen binds to the copper ions in hemocyanin, it causes the protein to change color to blue, indicating oxygenation. The Structure’s complexity and size can vary significantly between different species, adapting to their specific oxygen needs and environmental conditions.
Hemocyanin is not found in humans; instead, we use hemoglobin to transport oxygen through our bloodstream. Hemoglobin is an iron-containing protein in red blood cells, distinctly different from the copper-based hemocyanin found in some mollusks and arthropods. While hemocyanin turns blue when oxygenated, hemoglobin gives human blood its characteristic red color when it binds with oxygen. This difference highlights the diverse mechanisms that different organisms have evolved to manage essential functions like oxygen transport.
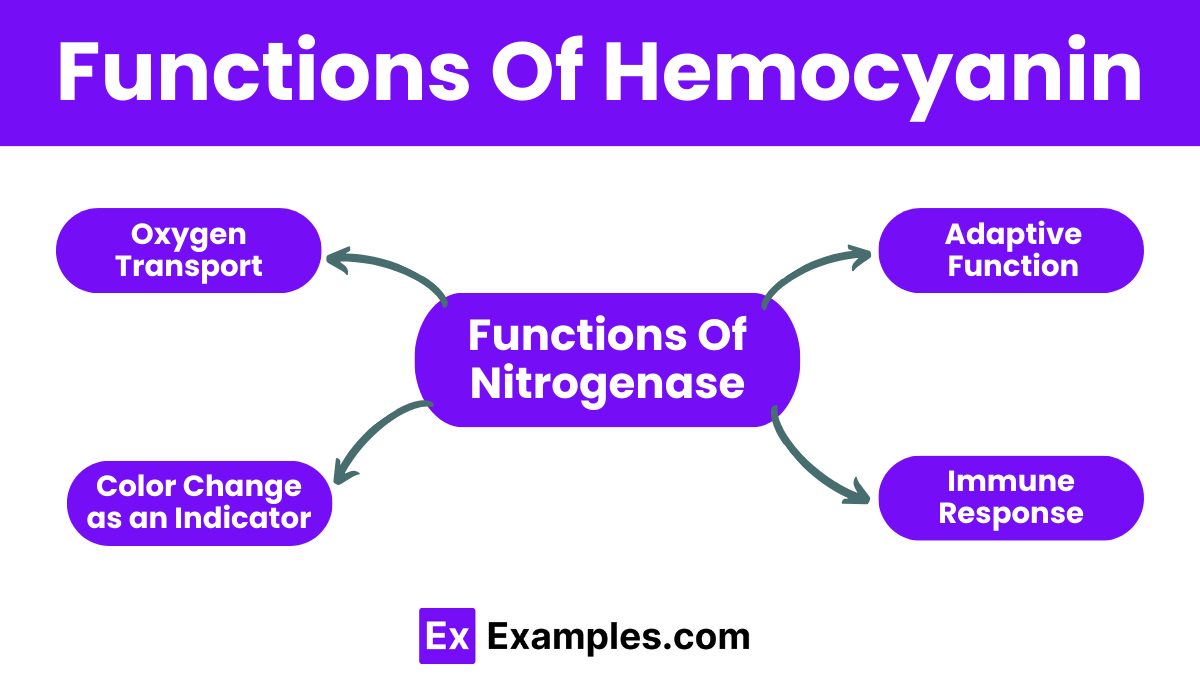
Hemocyanin primarily functions to transport oxygen from the respiratory surfaces of the organism to its tissues and cells. This is crucial for sustaining cellular respiration and energy production, especially in environments where oxygen might be scarce.
The presence of copper in hemocyanin allows it to change color upon binding with oxygen, turning from colorless to blue. This color change can serve as a visual indicator of oxygen saturation levels within the blood, providing insights into the respiratory efficiency of the organism.
The ability of hemocyanin to bind oxygen is especially advantageous in cold water environments where it can function more efficiently than hemoglobin. This adaptation helps organisms like mollusks and arthropods thrive in various ecological niches where temperature and oxygen availability vary.
Recent studies suggest that hemocyanin may also play a role in the immune response of some invertebrates. It is thought to help in the defense against pathogens by its ability to carry oxygen to immune cells quickly, enhancing their response to infection.
Hemocyanin is studied extensively in medical research due to its unique properties and structural differences from human hemoglobin. It offers insights into alternative oxygen transport mechanisms, which can lead to better understanding of respiratory diseases and potential new treatments for conditions involving oxygen uptake and utilization.
Due to its ability to bind oxygen and change color. Hemocyanin is explored for use in biotechnological applications, such as biosensors. These sensors could potentially detect gases or environmental changes based on the color change of hemocyanin. It providing a visual and effective means of analysis.
Hemocyanin from keyhole limpets is used as an immunostimulant in cancer vaccines. Its properties boost the immune response, making it a key component in developing therapeutic vaccines and treatments.
Research is also being conducted into the use of hemocyanin in environmental monitoring. Its sensitivity to changes in oxygen levels could make it useful for monitoring water quality. It particularly used in detecting hypoxic conditions that affect marine life health.
| Feature | Hemocyanin | Hemoglobin |
|---|---|---|
| Found in | Mollusks and arthropods | Vertebrates, including humans |
| Metal ion | Copper | Iron |
| Oxygen Binding | Binds oxygen directly to copper ions | Binds oxygen to iron in the heme group |
| Color change | Changes from colorless to blue when oxygenated | Changes from darker to bright red when oxygenated |
| Molecular Structure | Made up of multiple subunits, each with two copper atoms | Consists of four protein subunits, each with one iron atom |
| Function | Transports oxygen | Transports oxygen, also aids in carbon dioxide removal from the body |
| Thermal Adaptation | More efficient in colder, oxygen-rich environments | Functions effectively across a wide range of temperatures |
Hemocyanin is particularly effective at transporting oxygen in colder water environments where it maintains its ability to bind oxygen efficiently, unlike hemoglobin which can be less effective under similar conditions. This makes hemocyanin crucial for the survival of marine species living in such habitats.
The color change of hemocyanin from colorless to blue when oxygenated provides a visual indicator of the oxygen saturation in the blood. This can be useful for studies related to respiratory efficiency and the health status of the organisms.
Hemocyanin’s unique properties are being explored for use in biomedical applications, such as immunotherapy for cancer. Its ability to enhance the immune response makes it a promising agent in the development of vaccines and other therapeutic treatments.
The sensitivity of hemocyanin to oxygen levels can be utilized for environmental monitoring, especially in assessing water quality. Its response to varying oxygen concentrations can help detect pollution levels and the presence of toxic substances in aquatic environments.
Human blood without oxygen is a dark red or maroon color, due to deoxygenated hemoglobin.
Hemocyanin is not necessarily better, but more efficient in oxygen transport in cold, low-oxygen environments compared to hemoglobin.
Hemocyanin turns blue when oxygenated because the oxygen binds to copper ions in its structure, changing its color.
A scorpion’s blood is blue, due to the presence of hemocyanin, which contains copper that binds oxygen.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Hemocyanin is primarily found in which organisms?
Vertebrates
Mollusks and arthropods
Plants
Fungi
What metal ion is central to the hemocyanin molecule?
Iron
Copper
Zinc
Magnesium
What color is hemocyanin when it is oxygenated?
Red
Green
Blue
Yellow
How many copper ions are involved in oxygen binding in hemocyanin?
Two
One
Three
Four
Hemocyanin transports oxygen in which form of circulation?
Open
Closed
Pulmonary
Systemic
Which of the following is a characteristic of hemocyanin compared to hemoglobin?
Contains iron
Is red when oxygenated
Is blue when deoxygenated
Contains copper
Hemocyanin has a higher affinity for oxygen at:
Low pH
High pH
Low temperature
High temperature
Hemocyanin is similar to hemoglobin in that it:
Is found in all animals
Transports carbon dioxide
Transports oxygen
Is always red in color
Which type of bond is formed between oxygen and copper in hemocyanin?
Ionic bond
Covalent bond
Hydrogen bond
Coordinate covalent bond
Which animals are known to have blue blood due to hemocyanin?
Fish
Amphibians
Crustaceans
Birds
Before you leave, take our quick quiz to enhance your learning!

