What is a key characteristic of a homogeneous mixture?
The mixture has distinct layers
The composition is uniform throughout
The components are visible
The mixture can be separated by filtration


A homogeneous mixture is a combination of two or more substances that are uniformly distributed throughout the mixture, resulting in a single-phase system. In this type of mixture, the composition is consistent, and the individual components are not distinguishable by the naked eye or a microscope. Common examples of homogeneous mixtures include salt water, air, and vinegar. These mixtures can be separated into their individual components through physical means such as distillation or evaporation, but the distribution of each component remains even throughout the entire mixture.
A homogeneous mixture is a mixture where the components are uniformly distributed, resulting in a single-phase system with consistent composition throughout. Examples include salt water, air, and vinegar. These mixtures appear uniform and cannot be distinguished into their individual components visually.
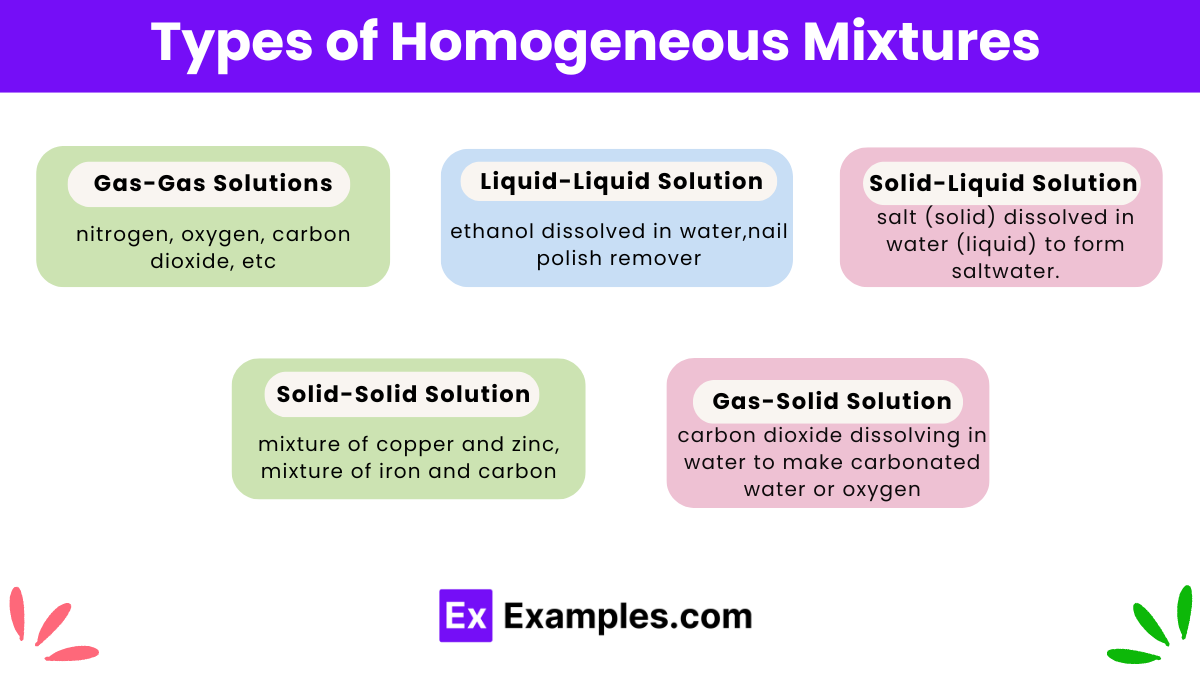
Homogeneous mixtures, also known as solutions, can be categorized into different types based on the state of the components and their interaction. Here are the main types of homogeneous mixtures:
Homogeneous mixtures, also known as solutions, exhibit several properties that distinguish them from heterogeneous mixtures. Here are the key properties of homogeneous mixtures:
Homogeneous mixtures have uniform properties, while heterogeneous mixtures do not.
Yes, they can be separated by techniques like filtration or distillation.
Yes, solutions where solute dissolves completely in solvent are homogeneous.
Phases refer to regions with uniform composition within the mixture.
By observing if it appears uniform throughout in terms of color and composition.
No, they do not show distinct boundaries between the mixed substances.
Yes, alloys like brass (copper and zinc) are homogeneous mixtures of metals.
Stirring, temperature, and solubility influence how substances mix uniformly.
Yes, if they have different compositions or properties in different parts.
Used in pharmaceuticals, food processing, and manufacturing for consistent product quality.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is a key characteristic of a homogeneous mixture?
The mixture has distinct layers
The composition is uniform throughout
The components are visible
The mixture can be separated by filtration
Which process is commonly used to separate components of a homogeneous mixture?
Filtration
Centrifugation
Distillation
Decantation
Which of the following is not a homogeneous mixture?
Air
Vinegar
Blood
Stainless steel
What type of mixture is a solution considered to be?
Heterogeneous mixture
Homogeneous mixture
Colloidal mixture
Suspension
Which statement best describes a homogeneous mixture?
It contains two or more substances that are visibly different
It has a variable composition
It is composed of a single phase
It can be easily separated into its components
What is an example of a gaseous homogeneous mixture?
Smoke
Air
Fog
Dust
Which of the following can be considered a homogeneous mixture?
Iron filings and sulfur powder
Sugar dissolved in water
Muddy water
Granite
In a homogeneous mixture, the ratio of solute to solvent can be described as:
Always equal
Variable but uniform throughout the mixture
Visible under a microscope
Non-existent
Which of the following is a characteristic of a homogeneous mixture?
Components can be seen with the naked eye
The mixture can be separated by physical means
The mixture has a uniform appearance and composition
The mixture forms separate layers
Which mixture is homogeneous?
Oil and vinegar
Water and ethanol
Water and sand
Smoke
Before you leave, take our quick quiz to enhance your learning!

