What is the chemical formula of hydrogen peroxide?
H₂O
H₂O₂
HO₂
H₃O₂


Hydrogen peroxide, a fascinating molecular compound in the world of chemistry, stands out for its simple, yet powerful formula: H₂O₂. Imagine water (H₂O), but with an extra oxygen atom, giving it incredible properties. This clear, liquid substance is more than just a chemical; it’s a versatile player in both science labs and everyday life. Known for its bleaching power and as a disinfectant, hydrogen peroxide is a staple in many experiments and household cleaning routines. Its unique structure and reactivity make it a topic of interest in chemistry classes, sparking curiosity among students eager to explore the molecular compounds that shape our world.
| Property | Value |
|---|---|
| Formula | H₂O₂ |
| Name | Hydrogen Peroxide |
| Alternate Names | Albone, Hydrogen Dioxide, Hydroperoxide |
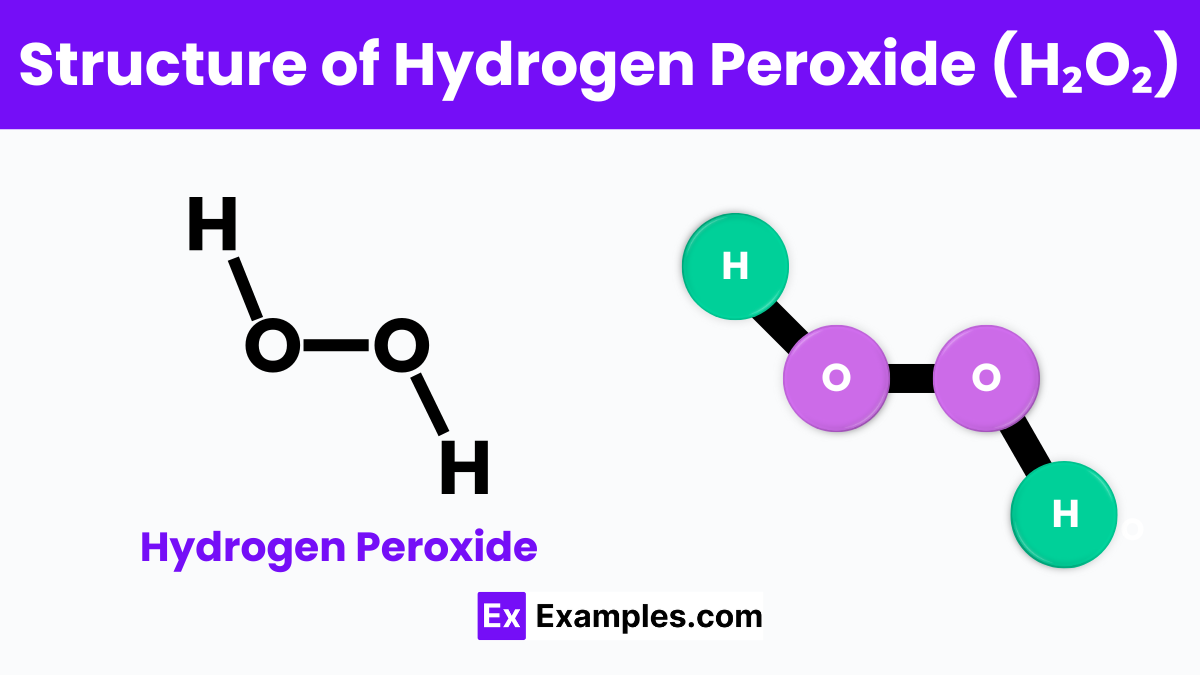
Imagine two friends holding hands with their other hand each holding a balloon; this is a bit like the structure of hydrogen peroxide (H₂O₂). In this molecule, the two “friends” are hydrogen atoms, and the “balloons” are oxygen atoms. They are connected in a way that forms a sort of V shape or a boomerang. The oxygen atoms are in the middle, closely bonded together, and each oxygen is also attached to a hydrogen atom. This unique arrangement makes hydrogen peroxide different from water (H₂O), where the hydrogens are both directly attached to the same oxygen. The structure of hydrogen peroxide gives it special properties, such as its ability to act as a powerful cleaning and bleaching agent. It’s this special shape that helps it break down into water and oxygen, making it useful for so many things around the house and in science.
Creating hydrogen peroxide in a lab involves a cool chemical reaction where you start with something called barium peroxide (BaO₂) and react it with sulfuric acid (H₂SO₄). Imagine mixing these two ingredients like combining baking soda and vinegar, but instead of a fizzy explosion, we get hydrogen peroxide (H₂O₂) and a by-product called barium sulfate (BaSO₄), which is a harmless, white powder that settles at the bottom. The chemical equation for this reaction looks like this:
In simpler terms, when barium peroxide meets sulfuric acid, they swap parts to form hydrogen peroxide and barium sulfate. The cool part is, the hydrogen peroxide can be collected and used for all its amazing properties, like cleaning wounds or bleaching hair, while the barium sulfate, being insoluble, is easily separated. This process shows the clever ways chemists play with different substances to create something useful and safe for everyday tasks.
| Property | Description |
|---|---|
| Appearance | It looks like water, being a clear, colorless liquid. |
| Odor | Hydrogen peroxide has a slightly sharp smell, similar to that of bleach. |
| Taste | It has a bitter taste, but it’s not safe to taste it! |
| Boiling Point | It boils at about 150.2°C (302.36°F), which is higher than water. |
| Freezing Point | It freezes into a pale blue solid at around -0.43°C (31.23°F), just below the freezing point of water. |
| Solubility | It dissolves well in water, making it easy to mix and use in various solutions. |
| Stability | It breaks down slowly into water (H2O) and oxygen (O2), especially when exposed to light, which is why it’s stored in dark bottles. |
As a mild antiseptic, it cleans wounds by releasing oxygen, which foams out dead cells and bacteria.
| Property | Value |
|---|---|
| CAS registry number | 7722-84-1 |
| Beilstein number | 3587191 |
| PubChem compound ID | 784 |
| SMILES identifier | OO |
| InChI identifier | InChI=1/H2O2/c1-2/h1-2H |
| RTECS number | MX0899500 |
| MDL number | MFCD00011333 |
| Property | Value |
|---|---|
| NFPA health rating | 3 |
| NFPA fire rating | 0 |
| NFPA reactivity rating | 1 |
| NFPA hazards | Oxidizing agent |
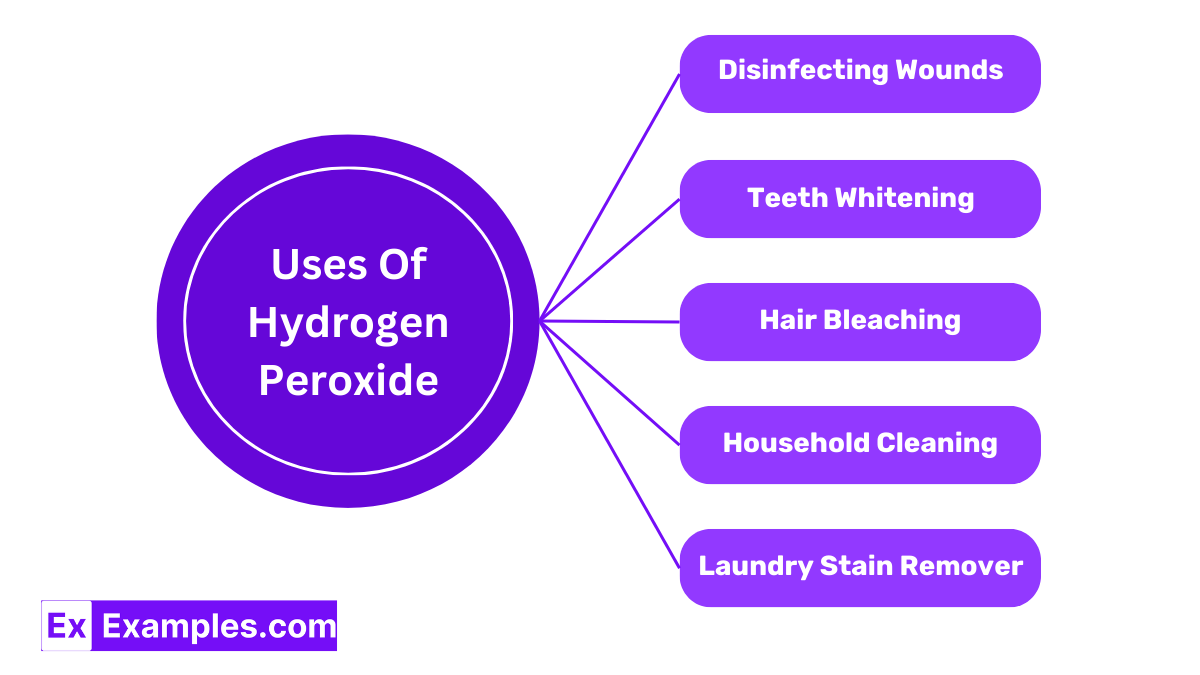
Hydrogen peroxide is used to clean cuts and scrapes. When applied, it foams up, helping to remove dirt and dead cells, and kills bacteria to prevent infection.
Many teeth whitening products contain hydrogen peroxide. It helps remove stains from the surface of your teeth, making them appear brighter and whiter.
A popular use of hydrogen peroxide is in hair bleaching products. It lightens the color of hair by breaking down the natural pigments, leading to a lighter appearance.
Thanks to its disinfecting properties, hydrogen peroxide is great for cleaning surfaces in your home. It’s especially useful in kitchens and bathrooms, where germs tend to gather.
Hydrogen peroxide can help remove tough stains on clothes. Applying it directly to stains like wine, blood, or sweat before washing can lead to cleaner laundry.
A diluted solution of hydrogen peroxide can help plants grow by adding extra oxygen to the soil, combating root rot, and acting as a natural pesticide.
In some cases, hydrogen peroxide is used to help purify water. It kills pathogens and breaks down harmful chemicals, making water safer to drink.
Hydrogen peroxide can cause skin irritation, disrupt wound healing, and bleach fabrics. Use cautiously and follow guidelines.
No, hydrogen peroxide and bleach are different. Hydrogen peroxide is a mild antiseptic and bleach is a stronger, chlorine-based cleaner.
In low concentrations, hydrogen peroxide is safe for skin but can cause irritation or damage if used improperly.
Using hydrogen peroxide on pimples can dry out skin and slow healing. Gentle acne treatments are recommended instead.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical formula of hydrogen peroxide?
H₂O
H₂O₂
HO₂
H₃O₂
Hydrogen peroxide is commonly used as a:
Fuel
Antiseptic
Lubricant
Coolant
In its pure form, hydrogen peroxide is:
A gas
A liquid
A solid
A plasma
What is the concentration of hydrogen peroxide commonly used in household disinfectants?
1%
3%
4%
6%
Hydrogen peroxide decomposes into:
Water and oxygen
Hydrogen and oxygen
Water and hydrogen
Hydrogen and nitrogen
Which catalyst can speed up the decomposition of hydrogen peroxide?
Sodium chloride
Potassium permanganate
Manganese dioxide
Copper sulfate
Hydrogen peroxide is used in which of the following applications?
Fuel for rockets
Preservative in food
Additive in gasoline
Antifreeze
Which property makes hydrogen peroxide a good bleaching agent?
Its acidity
Its basicity
Its oxidizing ability
Its reducing ability
What safety precaution should be taken when handling concentrated hydrogen peroxide?
Wear sunscreen
Use in a well-ventilated area
Mix with acids
Avoid all protective gear
Hydrogen peroxide can be broken down by which enzyme in living organisms?
Catalase
Amylase
Lipase
Protease
Before you leave, take our quick quiz to enhance your learning!

