What is the atomic number of Indium?
48
49
50
51
Indium, a lesser-known yet intriguing element, offers a wealth of educational opportunities. With its unique properties and applications, this guide is tailored to help educators bring the wonders of Indium into the classroom. From its role in technology to its place on the periodic table, we provide engaging examples and insights. Enhance your science curriculum with our Indium guide, designed to captivate and educate students about this fascinating metal.
What is Indium?
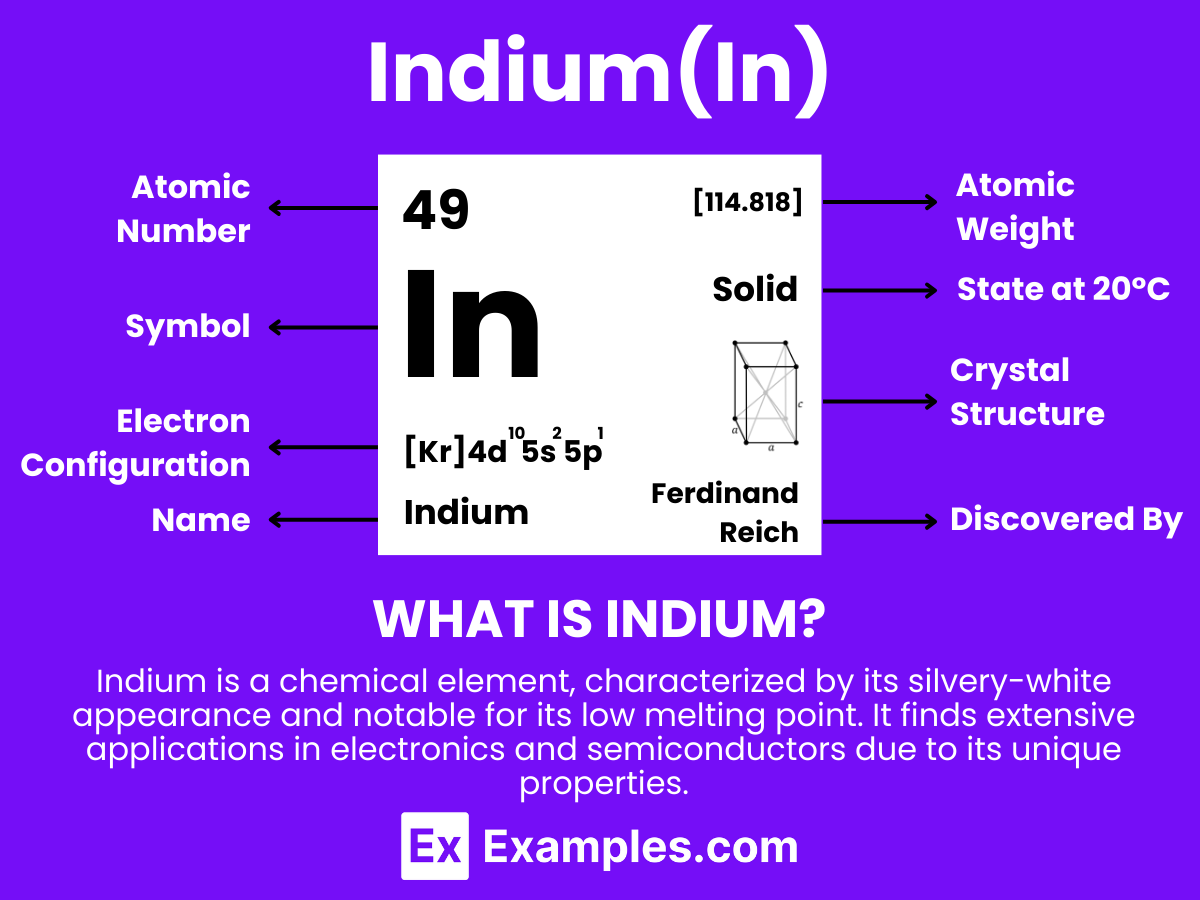
Indium is a chemical element with the symbol In and atomic number 49. It is a soft, malleable, and silvery-white metal that is commonly found in small amounts in zinc ores. Notably used in the production of LCD screens and solders, Indium has significant technological importance. Its low melting point and ability to adhere to glass make it valuable in various industrial applications. This element serves as an excellent teaching tool to illustrate concepts in chemistry and physics, particularly in discussions about metal properties and their roles in modern technology.
Indium metal (In) consists of atoms bonded together. Each indium atom has 49 protons in its nucleus and varying numbers of neutrons, depending on the isotope. In the indium solid, the atoms are closely packed in a metallic crystal lattice structure.
Atomic Level: Each indium atom (In) consists of 49 protons and a varying number of neutrons. Metallic Bonding: The indium atoms form metallic bonds with neighboring atoms, where outer electrons are delocalized and free to move throughout the metal lattice.
The bonding between the indium atoms is relatively strong due to metallic bonding, resulting in a solid metal with characteristic properties such as malleability, ductility, and conductivity. At room temperature, indium is a silvery-white, malleable metal with a relatively low melting point, making it useful in various applications such as electronics, soldering, and coatings.
| Property | Detail |
|---|---|
| Appearance | Silvery-white, lustrous metal |
| State at Room Temperature | Solid |
| Density | 7.31 g/cm³, relatively low density |
| Melting Point | 156.60 °C, one of the lowest for metals |
| Boiling Point | 2,072 °C |
| Electrical Conductivity | Highly conductive, used in electronics |
| Thermal Conductivity | Moderately high, efficient heat transfer |
| Malleability and Ductility | Highly malleable and ductile, easily formed |
| Crystal Structure | Tetragonal, typical of post-transition metals |
| Hardness | Relatively soft, can be cut with a knife |
| Sound Speed | Speed of sound in indium is about 1215 m/s |
| Reflectivity | Highly reflective, especially in thin films |
Indium is a relatively rare, post-transition metal known for its softness and malleability. Chemically, it shares properties with both its group members, gallium and thallium.
| Isotope | Natural Abundance | Half-Life | Decay Mode |
|---|---|---|---|
| In-113 | 4.3% | Stable | – |
| In-115 | 95.7% | 4.41 x 10¹⁴ years | Beta decay to tin-115 (Sn-115) |
Indium has two naturally occurring isotopes. Indium-115, the more abundant isotope, is mildly radioactive but with a very long half-life, making it practically stable for most practical purposes. The stability and abundance of these isotopes make indium a reliable element in various industrial and scientific applications.
Indium, a lustrous, silvery metal, has several vital applications in various industries due to its unique properties like malleability, ductility, and ability to form alloys. Here are the top five uses of Indium:
Indium is a key component in indium tin oxide (ITO), which is used in touch screens and liquid crystal displays (LCDs). ITO is a transparent conductor, making it ideal for controlling screen pixels in electronic devices like smartphones, tablets, and televisions.
Indium is used in solders and alloys due to its low melting point and ability not to corrode over time. It is particularly useful in lead-free solders and alloys with other metals to improve their thermal fatigue performance, making it essential in electronics manufacturing.
Indium phosphide (InP) and indium arsenide (InAs) are used in semiconductors for high-speed and high-frequency electronics. These compounds are vital in the production of diodes, transistors, and integrated circuits.
Due to its excellent thermal conductivity, Indium is used as a thermal interface material in heat sinks and heat exchangers. It helps in efficient heat dissipation in electronic devices, preventing overheating.
Indium is used in the production of thin-film solar cells. Indium gallium arsenide (InGaAs) and copper indium gallium selenide (CIGS) are used in photovoltaic cells for converting solar energy into electricity, contributing to sustainable energy solutions.
The commercial production of Indium primarily involves extracting it as a by-product from the processing of other metals, notably zinc. Indium is not usually found in its pure form but as a trace element in various minerals. The production process typically includes the following steps:
Indium, while valuable in various industrial applications, poses certain health risks when exposure occurs, particularly in occupational settings. Understanding these health effects is crucial for ensuring safety and implementing appropriate protective measures.
Indium’s environmental impact, particularly due to mining and industrial use, is an area of growing concern, emphasizing the need for sustainable practices and effective waste management.
Indium is generally considered non-toxic to humans in small quantities. However, prolonged exposure or ingestion of large amounts can lead to health concerns, including gastrointestinal irritation and potential organ damage. Proper handling and disposal practices are recommended to minimize any potential risks associated with indium exposure
Indium finds versatile applications due to its unique properties. It is commonly used in the production of electronic components like semiconductors and liquid crystal displays (LCDs). Additionally, it serves as a crucial component in alloys, solders, and thin-film coatings, contributing to advancements in technology and various industrial processes
Yes, you can touch indium with your bare hands. Indium is a solid metal at room temperature and is generally safe to handle. However, it’s always advisable to wash your hands afterward to remove any potential contaminants, as with handling any other metallic substances.
In summary, indium is a versatile metal with unique properties that make it valuable in various industries. Its malleability, conductivity, and low melting point contribute to its widespread use in electronics, soldering, and coatings. Understanding the atomic and molecular structure of indium provides insight into its behavior and applications, highlighting its importance in modern technology and manufacturing processes.

Indium, a lesser-known yet intriguing element, offers a wealth of educational opportunities. With its unique properties and applications, this guide is tailored to help educators bring the wonders of Indium into the classroom. From its role in technology to its place on the periodic table, we provide engaging examples and insights. Enhance your science curriculum with our Indium guide, designed to captivate and educate students about this fascinating metal.
What is Indium?
Indium is a chemical element with the symbol In and atomic number 49. It is a soft, malleable, and silvery-white metal that is commonly found in small amounts in zinc ores. Notably used in the production of LCD screens and solders, Indium has significant technological importance. Its low melting point and ability to adhere to glass make it valuable in various industrial applications. This element serves as an excellent teaching tool to illustrate concepts in chemistry and physics, particularly in discussions about metal properties and their roles in modern technology.
Formula: In
Composition: A single indium atom.
Bond Type: Indium typically forms covalent or metallic bonds, utilizing its three valence electrons.
Molecular Structure: Soft, malleable, and silvery-white, with a tetragonal crystal structure.
Electron Configuration: 49 electrons, with the configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p¹.
Significance: Important in the manufacture of touchscreens, liquid crystal displays, and solders.
Role in Chemistry: Indium’s unique properties make it valuable in materials science and technological applications.
Indium metal (In) consists of atoms bonded together. Each indium atom has 49 protons in its nucleus and varying numbers of neutrons, depending on the isotope. In the indium solid, the atoms are closely packed in a metallic crystal lattice structure.
Atomic Level: Each indium atom (In) consists of 49 protons and a varying number of neutrons. Metallic Bonding: The indium atoms form metallic bonds with neighboring atoms, where outer electrons are delocalized and free to move throughout the metal lattice.
The bonding between the indium atoms is relatively strong due to metallic bonding, resulting in a solid metal with characteristic properties such as malleability, ductility, and conductivity. At room temperature, indium is a silvery-white, malleable metal with a relatively low melting point, making it useful in various applications such as electronics, soldering, and coatings.
Property | Detail |
|---|---|
Appearance | Silvery-white, lustrous metal |
State at Room Temperature | Solid |
Density | 7.31 g/cm³, relatively low density |
Melting Point | 156.60 °C, one of the lowest for metals |
Boiling Point | 2,072 °C |
Electrical Conductivity | Highly conductive, used in electronics |
Thermal Conductivity | Moderately high, efficient heat transfer |
Malleability and Ductility | Highly malleable and ductile, easily formed |
Crystal Structure | Tetragonal, typical of post-transition metals |
Hardness | Relatively soft, can be cut with a knife |
Sound Speed | Speed of sound in indium is about 1215 m/s |
Reflectivity | Highly reflective, especially in thin films |
Indium is a relatively rare, post-transition metal known for its softness and malleability. Chemically, it shares properties with both its group members, gallium and thallium.
Reactivity: Indium is not very reactive at room temperature. It does not react with water, but it will oxidize over time upon exposure to air, forming an indium(III) oxide layer on its surface.
Equation: 4In+3O₂ →2In₂O₃
Acid Reaction: Indium reacts with acids, such as hydrochloric acid, to form indium(III) chloride and hydrogen gas.
Equation: 2In+6HCl→2InCl₃+3H₂
Alloys: Indium forms alloys with many metals and significantly lowers the melting point of the alloy, which is crucial in applications like fusible alloys, solders, and thermal interface materials.
Oxidation States: Indium predominantly shows the +3 oxidation state. However, it can also form the less stable +1 state in some compounds.
Compounds: Indium forms various compounds, such as indium tin oxide (ITO), which is used in touch screens and liquid crystal displays due to its electrical conductivity and optical transparency.
Equation for ITO Synthesis: In2O₃+SnO₂ →ITO
Isotopes: Indium has two naturally occurring isotopes, 113In and 115In being mildly radioactive but with a very long half-life, posing negligible radiological hazards.
Indium(III) Oxide (In₂O₃)
Equation: 4In+3O₂→2In2O₃₄
Indium(III) oxide is used in touch screens and flat-panel displays due to its electrical conductivity and transparency.
Indium Tin Oxide (ITO)
Equation: In2O₃+SnO₂→ITO
A mixture of indium(III) oxide and tin oxide, ITO is crucial for making transparent conductive coatings for touchscreens and solar cells.
Indium(III) Chloride (InCl₃)
Equation: In+3Cl₂→2InCl₃
Used as a starting material for the synthesis of other indium compounds, indium(III) chloride is also used in organic synthesis and electronics.
Indium(III) Sulfide (In2S₃)
Equation: 2In+3S→In2S₃
This compound is used in the manufacture of semiconductors and as a photocatalyst in the splitting of water.
Indium Phosphide (InP)
Equation: In+P→InP
Indium phosphide is used in high-speed and high-frequency electronics due to its superior electron velocity.
Indium Antimonide (InSb)
Equation: In+Sb→InSb
A semiconductor used in infrared detectors and Hall effect devices, known for its rapid electron transport properties.
Isotope | Natural Abundance | Half-Life | Decay Mode |
|---|---|---|---|
In-113 | 4.3% | Stable | – |
In-115 | 95.7% | 4.41 x 10¹⁴ years | Beta decay to tin-115 (Sn-115) |
Indium has two naturally occurring isotopes. Indium-115, the more abundant isotope, is mildly radioactive but with a very long half-life, making it practically stable for most practical purposes. The stability and abundance of these isotopes make indium a reliable element in various industrial and scientific applications.
Indium, a lustrous, silvery metal, has several vital applications in various industries due to its unique properties like malleability, ductility, and ability to form alloys. Here are the top five uses of Indium:
Indium is a key component in indium tin oxide (ITO), which is used in touch screens and liquid crystal displays (LCDs). ITO is a transparent conductor, making it ideal for controlling screen pixels in electronic devices like smartphones, tablets, and televisions.
Indium is used in solders and alloys due to its low melting point and ability not to corrode over time. It is particularly useful in lead-free solders and alloys with other metals to improve their thermal fatigue performance, making it essential in electronics manufacturing.
Indium phosphide (InP) and indium arsenide (InAs) are used in semiconductors for high-speed and high-frequency electronics. These compounds are vital in the production of diodes, transistors, and integrated circuits.
Due to its excellent thermal conductivity, Indium is used as a thermal interface material in heat sinks and heat exchangers. It helps in efficient heat dissipation in electronic devices, preventing overheating.
Indium is used in the production of thin-film solar cells. Indium gallium arsenide (InGaAs) and copper indium gallium selenide (CIGS) are used in photovoltaic cells for converting solar energy into electricity, contributing to sustainable energy solutions.
The commercial production of Indium primarily involves extracting it as a by-product from the processing of other metals, notably zinc. Indium is not usually found in its pure form but as a trace element in various minerals. The production process typically includes the following steps:
Ore Processing: Indium is most commonly obtained from the ores of zinc, lead, and tin. During the ore processing of these metals, Indium is also extracted.
Concentration: The process of concentration involves crushing and grinding the ore, followed by flotation and separation to increase the Indium concentration.
Refining: The concentrated Indium is then subjected to a refining process. It involves roasting the ore, which converts the Indium to the oxide form, followed by reduction with carbon to obtain the metal.
Purification: The crude Indium is further purified using techniques like electrolysis or zone refining, where impurities are removed, and the metal is brought to a high degree of purity.
Casting: Finally, the purified Indium is cast into ingots, bars, or other forms based on industrial requirements.
Indium, while valuable in various industrial applications, poses certain health risks when exposure occurs, particularly in occupational settings. Understanding these health effects is crucial for ensuring safety and implementing appropriate protective measures.
Respiratory Issues: The most significant health risk associated with Indium is its impact on the respiratory system. Prolonged inhalation of indium compounds, especially indium tin oxide (ITO), can lead to serious lung diseases, including pulmonary alveolitis, pneumonitis, and interstitial lung disease.
Indium Lung: A specific condition known as “Indium Lung” has been identified in workers exposed to indium compounds. Symptoms include coughing, dyspnea (difficulty breathing), and chest tightness. In severe cases, it can lead to pulmonary fibrosis, a potentially fatal scarring of the lungs.
Skin and Eye Irritation: Indium compounds can cause skin and eye irritation upon contact. While not deeply penetrating, these irritations can be uncomfortable and lead to dermatitis.
Limited Data on Carcinogenicity: Currently, there is limited data regarding indium’s carcinogenicity. However, given its effects on the lungs, continuous monitoring and research are essential.
Preventive Measures: To mitigate these health risks, workplaces should enforce safety protocols such as proper ventilation, personal protective equipment (PPE), and regular health check-ups for workers handling indium.
Indium’s environmental impact, particularly due to mining and industrial use, is an area of growing concern, emphasizing the need for sustainable practices and effective waste management.
Mining Impact: The extraction of indium, often as a byproduct of zinc, lead, and copper mining, can lead to environmental degradation. This includes landscape disruption, water pollution, and the release of toxic substances.
Water Pollution: The release of indium compounds into water bodies during mining and industrial processes can affect aquatic ecosystems. These compounds may be toxic to aquatic organisms, disrupting food chains and water quality.
Soil Contamination: Indium can accumulate in soils near mining and processing sites, potentially affecting plant growth and soil health. This contamination can have long-term effects on local ecosystems and agriculture.
Electronic Waste: A significant concern is the disposal of electronic devices containing indium, such as LCD screens. If not properly recycled, indium can contribute to environmental pollution.
Recycling and Management: Efficient recycling of indium from electronic waste is crucial for reducing environmental impact. This includes developing more sustainable mining practices and promoting the circular use of indium in the industry.
Indium is generally considered non-toxic to humans in small quantities. However, prolonged exposure or ingestion of large amounts can lead to health concerns, including gastrointestinal irritation and potential organ damage. Proper handling and disposal practices are recommended to minimize any potential risks associated with indium exposure
Indium finds versatile applications due to its unique properties. It is commonly used in the production of electronic components like semiconductors and liquid crystal displays (LCDs). Additionally, it serves as a crucial component in alloys, solders, and thin-film coatings, contributing to advancements in technology and various industrial processes
Indium is one of the rarest naturally occurring elements on Earth, making up only a few parts per million in the Earth’s crust.
It remains liquid over a wide temperature range, allowing it to be used in specialized alloys and as a heat-transfer medium.
Indium-tin oxide is a transparent and conductive material used in touchscreens, solar panels, and liquid crystal displays
Yes, you can touch indium with your bare hands. Indium is a solid metal at room temperature and is generally safe to handle. However, it’s always advisable to wash your hands afterward to remove any potential contaminants, as with handling any other metallic substances.
In summary, indium is a versatile metal with unique properties that make it valuable in various industries. Its malleability, conductivity, and low melting point contribute to its widespread use in electronics, soldering, and coatings. Understanding the atomic and molecular structure of indium provides insight into its behavior and applications, highlighting its importance in modern technology and manufacturing processes.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Indium?
48
49
50
51
What is the chemical symbol for Indium?
In
Id
Im
Ir
Indium belongs to which group in the periodic table?
Group 12
Group 13
Group 14
Group 15
What is the most common oxidation state of Indium?
+1
+2
+3
+4
Indium is often used in which type of display technology?
LED
LCD
CRT
Plasma
What is the primary mineral source of Indium?
Sphalerite
Bauxite
Hematite
Galena
What is the melting point of Indium?
156.6°C
231.9°C
327.5°C
660.3°C
Indium forms alloys with which metal to create low-melting-point alloys?
Aluminum
Bismuth
Copper
Nickel
Which property of Indium makes it useful for creating seals in cryogenics?
High melting point
High density
Ductility
Low reactivity
In which year was Indium discovered?
1800
1863
1901
1937
Before you leave, take our quick quiz to enhance your learning!

