What is the primary force that holds ionic compounds together?
Covalent bonds
Ionic bonds
Metallic bonds
Hydrogen bonds
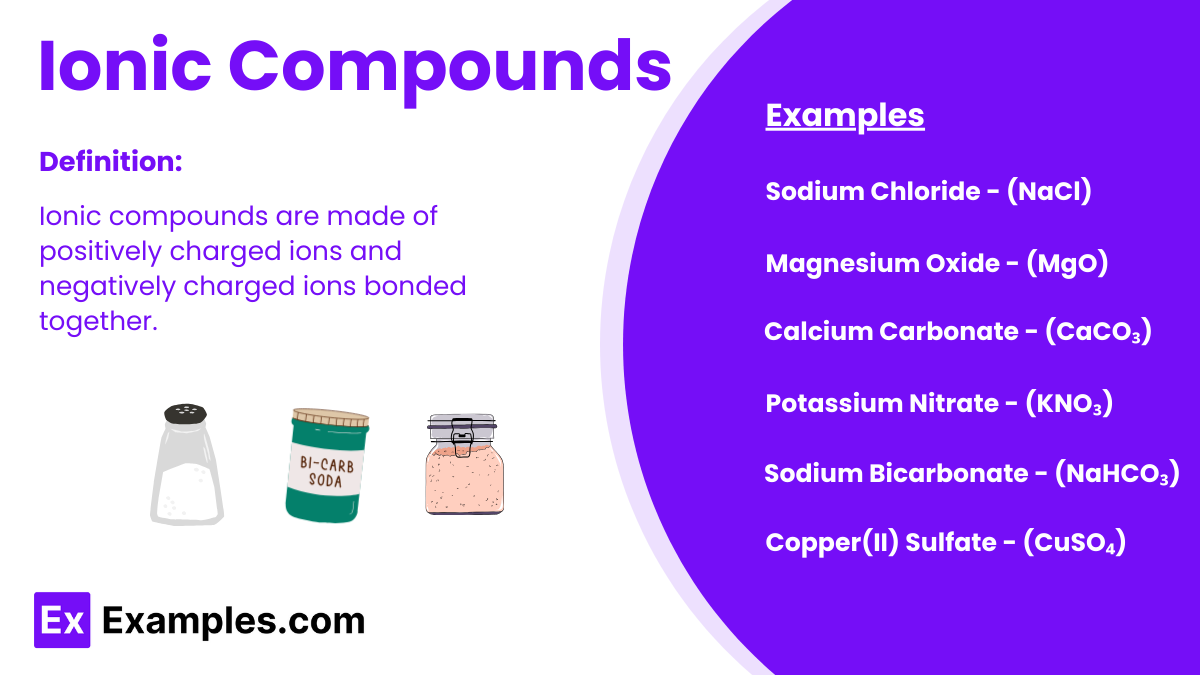
A lot of chemists create compounds that we utilize in our everyday lives due to their accessibility and affordability. Most of these compounds are stable and will not cause any sudden danger due to their ionic bonds. Because of their bonds, these compounds are known as ionic compounds.
Ionic compounds are specific types of compounds that are generated when a chemist uses an ionic bond to form the said compound. This compound is very stable and is a direct juxtaposition of the covalent compound formed from covalent bonds.
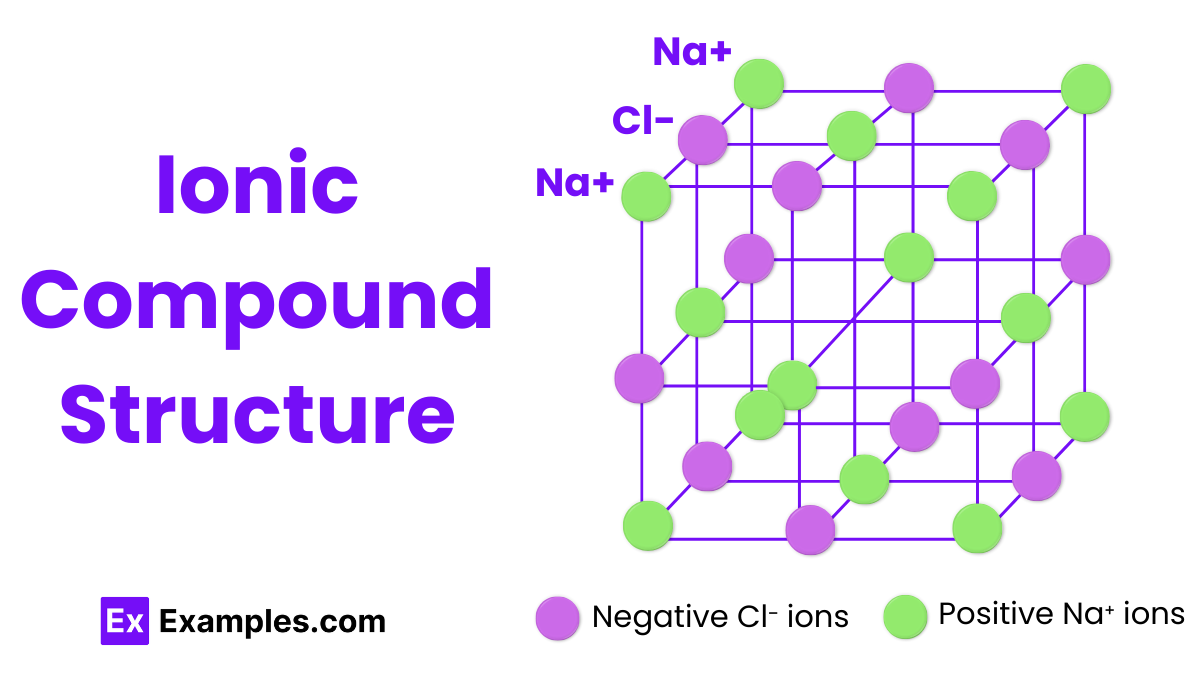
The structure of ionic compounds is characterized by a crystalline lattice arrangement. In this structure, positively charged ions (cations) and negatively charged ions (anions) are held together by strong electrostatic forces known as ionic bonds. This lattice extends in a repeating pattern throughout the solid, maximizing the attraction between oppositely charged ions while minimizing the repulsion between like-charged ions, resulting in a stable, solid structure.
Ionic character refers to the degree to which a chemical bond between two atoms has the characteristics of an ionic bond. An ionic bond is a type of chemical bond formed through the electrostatic attraction between oppositely charged ions. Ions are atoms that have gained or lost electrons, resulting in a net positive or negative charge. Ionic bonds are typically formed between metals and non-metals.
The ionic character of a bond can be estimated using the Pauling scale of electronegativity. One way to approximate the ionic character of a bond is by using the formula:
Where:
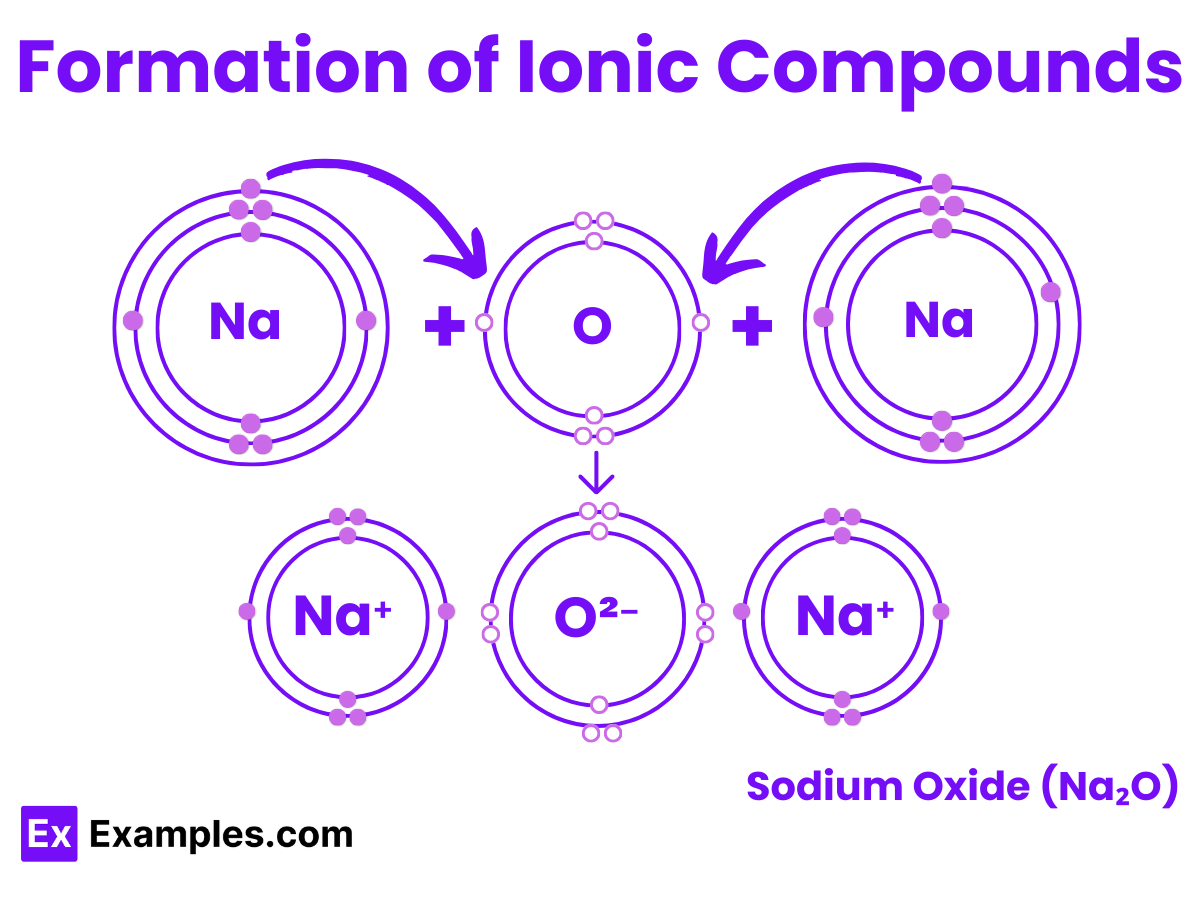
For example, the interaction between sodium and oxygen. The sodium atom has one electron in its outermost shell. By giving up this single electron from its outer shell, the shell beneath it becomes the new outermost shell, which has a full stable octet. The nucleus of the sodium atom still possesses eleven protons, but the number of electrons has become ten. This results in a net positive charge on the sodium atom, producing a sodium cation Na⁺.
Conversely, the oxygen atom has six electrons in its outermost shell. Hence, it requires two electrons to complete its octet. It can accept these two electrons from two sodium atoms to achieve a stable electronic configuration. Since one oxygen atom can gain two electrons and each sodium atom loses one, two atoms of sodium are necessary to combine with one atom of oxygen to form sodium oxide, Na₂O.
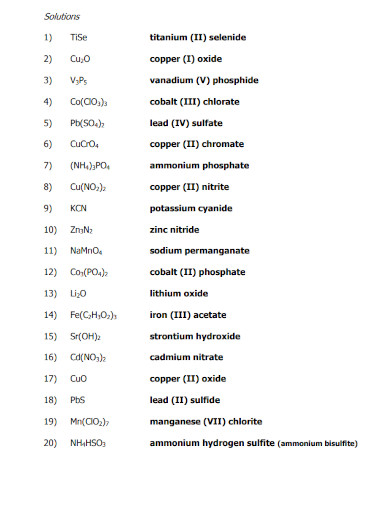
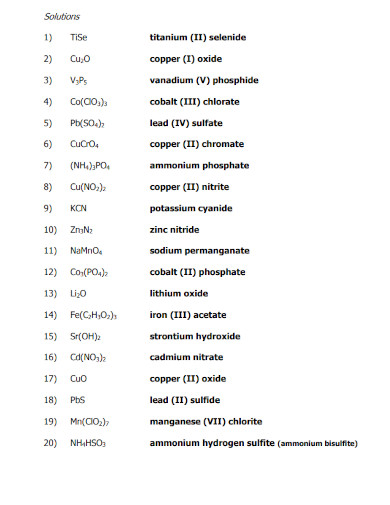
Ionic compounds have their own naming conventions that scientists and chemists use to easily identify the components of said compounds. This is a very important concept to understand when one wants to model ionic compounds. Not only that but the components will also dictate the physical properties of the ionic compounds.
Begin by figuring out all the components of all the ions found in the ionic compound. This will help you easily identify the name of the cations and anions without getting confused.
The cations are the ions found in the ionic compound that hold the positive charge. If there is only one cation, you must identify the single cation in the compound. But if there are multiple cations or a compound acting as a cation then you must figure out all of their names. For example, if the Cation is H then it is simply going to be named Hydrogen. But if the cation is (NH3) it will be named ammonium. Note that there are special cases for specific elements, which are iron (Fe), copper (Cu), tin (Sn), lead(Pb) silver (Ag), chromium (Cr), and gold (Au).
The anion is the negative charge that will pair up with the cation resulting in a net neutral charge for the compound. The anion will be simply named the element with the suffix -ide (for monoatomic ions) and -ate (for polyatomic ions).
After you have supplied the names of all the ions in the compound, you must arrange them in such a way that cations precede the anions. An example of this would be sodium chloride (NaCl) where sodium (Na) comes before the chlorine ion (Cl).
Ionic compounds exhibit a distinctive set of physical properties due to the strong electrostatic forces of attraction between the oppositely charged ions in their lattice structure. Below is a table that describes these properties in detail:
| Property | Description |
|---|---|
| High Melting and Boiling Points | Ionic compounds typically have high melting and boiling points. This is due to the strong ionic bonds that require significant amounts of energy to break. |
| Hardness and Brittleness | Ionic compounds are generally hard because of the strong binding forces between the ions. However, they are also brittle and can shatter when subjected to a force. |
| Electrical Conductivity | In the solid state, ionic compounds do not conduct electricity because the ions are fixed in place. However, when melted or dissolved in water, they conduct electricity due to the mobility of ions. |
| Solubility in Water | Many ionic compounds are soluble in water. The polarity of water molecules enables them to surround and separate the ions, leading to dissolution. |
| Density | Ionic compounds tend to have high densities due to the close packing of ions in their lattice structure. |
Ionic compounds are chemical compounds made up of ions held together by ionic bonds. These ions are atoms or molecules that have gained or lost electrons, resulting in a net charge. The formation of ionic compounds is primarily between metals and non-metals. Here are some key characteristics of ionic compounds:
Ionic compounds form a crystalline lattice structure. This regular, repeating pattern of ions results in the formation of solid crystals at room temperature. The structure is highly organized, allowing ionic compounds to have high melting and boiling points.
Due to the strong electrostatic forces of attraction between the oppositely charged ions, ionic compounds typically have high melting and boiling points. This means they are solid at room temperature and require a significant amount of energy to change into a liquid or gas.
In solid form, ionic compounds do not conduct electricity because the ions are fixed in place within the crystal lattice and cannot move freely. However, when dissolved in water or melted into a liquid, ionic compounds conduct electricity. This is because the ions are free to move and can carry charge through the solution or molten liquid.
Many ionic compounds are soluble in water. The polarity of water molecules allows them to interact with the positive and negative ions in an ionic compound, facilitating the compound’s dissolution. The extent of solubility varies among different ionic compounds, depending on the strength of the ionic bonds and the specific ions involved.
Despite their strength, ionic compounds are brittle. When force is applied, the crystal lattice can be distorted, causing ions of the same charge to be brought closer together. This repulsion between like-charged ions can cause the crystal to shatter or break apart.
Ionic compounds typically form when metals react with non-metals. Metals tend to lose electrons to achieve a stable electron configuration, becoming positively charged ions (cations). Non-metals tend to gain these electrons, becoming negatively charged ions (anions). The electrostatic attraction between these oppositely charged ions leads to the formation of ionic compounds.
The formation of ionic compounds is usually exothermic, meaning it releases energy. This release of energy occurs because the formation of strong ionic bonds between the ions releases more energy than is required to separate the electrons from the atoms.
One of the biggest properties of an ionic compound is its ability to conduct electricity in various states of matter. This means that if the ionic compound is in an aqueous form or is in a molten state, then it will be able to easily and effectively conduct electricity through its body. This quality exists in aqueous and molten ionic compounds because of the free-flow movement of all the ions in the compound. But if the ionic compound is in a solid state then it cannot conduct electricity due to the static position of all the ions in the compound.
Ionic compounds are very stable and will require great effort to split the atoms apart through dissolution. The ionic bond of the atoms in the compound causes the neutralization of all the charges of the compounds, which creates a very stable bond. Examples of ionic compounds used in our everyday life are table salt (sodium chloride or NaCl), baking soda (Na?2HCO^3 or sodium bicarbonate), and Calcium nitride (Ca3N2). Most of these ionic compounds are stable and will not immediately separate or create a new mixture upon contact with air and water.
Yes, ionic compounds can generally become soluble when immersed in a polar substance. This is due to their ionic bonds and their neutral charges. But not all ionic compounds are safe to mix with water. Calcium nitride has a reaction with water that creates an invisible flammable gas that can irritate and corrode one’s lungs, which will inadvertently lead to lung disease or damage. Not only that, but calcium nitride also creates a gas that can spontaneously combust, after reacting with water molecules in the atmosphere. This means that most ionic compounds are water-soluble, but not all can be safely mixed with water.
Ionic compounds are a type of mixture and compound that people create when they mix two or more atoms or substances that will end up with an ionic bond. These types of compounds have their own nomenclature. Future scientists and chemists should understand and know the importance of ionic compounds and how to create and name said compounds in a lab.

A lot of chemists create compounds that we utilize in our everyday lives due to their accessibility and affordability. Most of these compounds are stable and will not cause any sudden danger due to their ionic bonds. Because of their bonds, these compounds are known as ionic compounds.
Ionic compounds are specific types of compounds that are generated when a chemist uses an ionic bond to form the said compound. This compound is very stable and is a direct juxtaposition of the covalent compound formed from covalent bonds.

The structure of ionic compounds is characterized by a crystalline lattice arrangement. In this structure, positively charged ions (cations) and negatively charged ions (anions) are held together by strong electrostatic forces known as ionic bonds. This lattice extends in a repeating pattern throughout the solid, maximizing the attraction between oppositely charged ions while minimizing the repulsion between like-charged ions, resulting in a stable, solid structure.
Ionic character refers to the degree to which a chemical bond between two atoms has the characteristics of an ionic bond. An ionic bond is a type of chemical bond formed through the electrostatic attraction between oppositely charged ions. Ions are atoms that have gained or lost electrons, resulting in a net positive or negative charge. Ionic bonds are typically formed between metals and non-metals.
The ionic character of a bond can be estimated using the Pauling scale of electronegativity. One way to approximate the ionic character of a bond is by using the formula:
% Ionic Character=(1−e−0.25(Δχ)2)×100%
Where:
Δχ is the difference in electronegativity between the two atoms involved in the bond.
The exponential is to the base e, which is approximately equal to 2.71828.

For example, the interaction between sodium and oxygen. The sodium atom has one electron in its outermost shell. By giving up this single electron from its outer shell, the shell beneath it becomes the new outermost shell, which has a full stable octet. The nucleus of the sodium atom still possesses eleven protons, but the number of electrons has become ten. This results in a net positive charge on the sodium atom, producing a sodium cation Na⁺.
Conversely, the oxygen atom has six electrons in its outermost shell. Hence, it requires two electrons to complete its octet. It can accept these two electrons from two sodium atoms to achieve a stable electronic configuration. Since one oxygen atom can gain two electrons and each sodium atom loses one, two atoms of sodium are necessary to combine with one atom of oxygen to form sodium oxide, Na₂O.

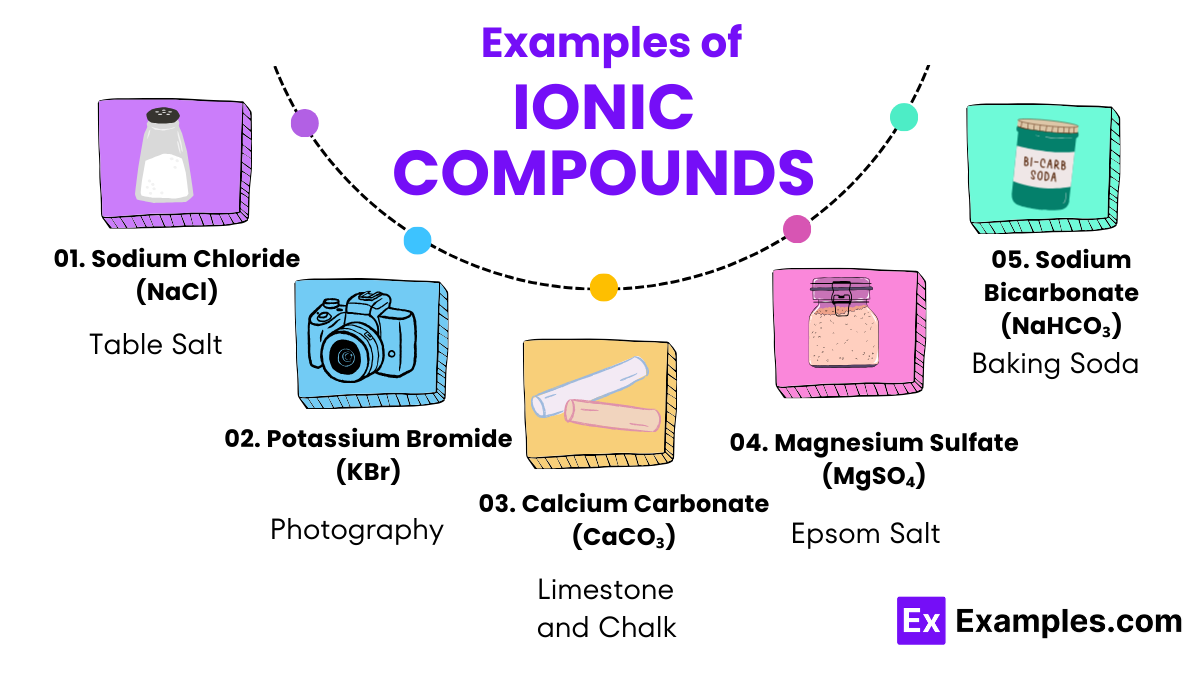
Sodium Chloride (Table Salt)
Formation Equation: Na + Cl₂ → 2NaCl
Sodium (Na) reacts with chlorine (Cl₂) to form sodium chloride (NaCl), widely used as table salt for seasoning and preserving food.
Potassium Bromide (Used in Photography)
Formation Equation: 2K + Br₂ → 2KBr
Potassium (K) reacts with bromine (Br₂) to produce potassium bromide (KBr), which is used in photography for its properties as a light-sensitive compound.
Calcium Carbonate (Found in Limestone and Chalk)
Formation Equation: Ca²⁺ + CO₃²⁻ → CaCO₃
Calcium ions (Ca²⁺) combine with carbonate ions (CO₃²⁻) to form calcium carbonate (CaCO₃), employed as a key ingredient in cement and chalk, and widely used in agriculture to amend soil pH.
Magnesium Sulfate (Epsom Salt)
Formation Equation: Mg + H₂SO₄ → MgSO₄ + H₂
Magnesium (Mg) reacts with sulfuric acid (H₂SO₄) to produce magnesium sulfate (MgSO₄), known as Epsom salt, used in bath salts for relaxation and in agriculture to correct magnesium deficiency in soils.
Sodium Bicarbonate (Baking Soda)
Formation Equation: Na⁺ + HCO₃⁻ → NaHCO₃
Sodium ions (Na⁺) react with bicarbonate ions (HCO₃⁻) to create sodium bicarbonate (NaHCO₃), commonly known as baking soda, used in baking, cleaning, and as an antacid.
Potassium Chloride (Used in Fertilizers)
Formation Equation: K + Cl₂ → 2KCl
Potassium (K) reacts with chlorine (Cl₂) to form potassium chloride (KCl), widely used in fertilizers to provide the essential nutrient potassium to plants.
Copper(II) Sulfate (Used in Fungicides)
Formation Equation: Cu + 2H₂SO₄ → CuSO₄ + SO₂ + 2H₂O
Copper (Cu) reacts with sulfuric acid (H₂SO₄) to produce copper(II) sulfate (CuSO₄), used in fungicides for its antimicrobial properties and in various industrial applications.
Iron(II) Sulfide (Occurs in Pyrite)
Formation Equation: Fe + S → FeS
Iron (Fe) reacts with sulfur (S) to form iron(II) sulfide (FeS), which naturally occurs in the mineral pyrite, known for its metallic luster and gold color.
Calcium Sulfate (Known as Gypsum)
Formation Equation: Ca²⁺ + SO₄²⁻ → CaSO₄
Calcium ions (Ca²⁺) react with sulfate ions (SO₄²⁻) to form calcium sulfate (CaSO₄), widely used in the construction industry as gypsum for making drywall and plaster.
Silver Nitrate (Used in Photography and Medicine)
Formation Equation: Ag + HNO₃ → AgNO₃ + ½H₂
Silver (Ag) reacts with nitric acid (HNO₃) to produce silver nitrate (AgNO₃), utilized in photography for developing photographs and in medicine for its antiseptic and cauterizing properties.
christs-hospital.lincs.sch.uk
Details
File Format
Size: 34 KB

csudh.edu
Details
File Format
Size: 53 KB

chemistry.christianedgar.com
Details
File Format
Size: 45 KB

assets.ctfassets.net
Details
File Format
Size: 39 KB
everettcc.edu
Details
File Format
Size: 43 KB

2012books.lardbucket.org
Details
File Format
Size: 70 KB

west-windsor-plainsboro.k12.nj.us
Details
File Format
Size: 37 KB

franklinboe.org
Details
File Format
Size: 70 KB

eips.ca
Details
File Format
Size: 52 KB
sfponline.org
Details
File Format
Size: 43 KB

hamilton-local.k12.oh.us
Details
File Format
Size: 90 KB
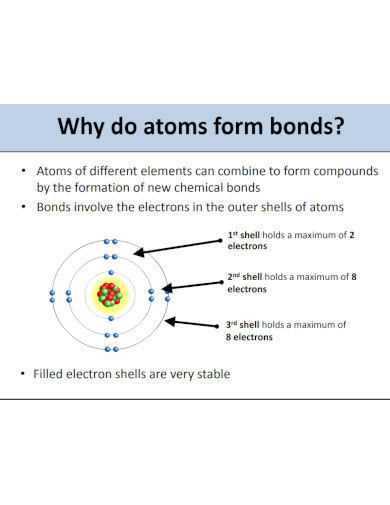
Ionic compounds have their own naming conventions that scientists and chemists use to easily identify the components of said compounds. This is a very important concept to understand when one wants to model ionic compounds. Not only that but the components will also dictate the physical properties of the ionic compounds.
Begin by figuring out all the components of all the ions found in the ionic compound. This will help you easily identify the name of the cations and anions without getting confused.
The cations are the ions found in the ionic compound that hold the positive charge. If there is only one cation, you must identify the single cation in the compound. But if there are multiple cations or a compound acting as a cation then you must figure out all of their names. For example, if the Cation is H then it is simply going to be named Hydrogen. But if the cation is (NH3) it will be named ammonium. Note that there are special cases for specific elements, which are iron (Fe), copper (Cu), tin (Sn), lead(Pb) silver (Ag), chromium (Cr), and gold (Au).
The anion is the negative charge that will pair up with the cation resulting in a net neutral charge for the compound. The anion will be simply named the element with the suffix -ide (for monoatomic ions) and -ate (for polyatomic ions).
After you have supplied the names of all the ions in the compound, you must arrange them in such a way that cations precede the anions. An example of this would be sodium chloride (NaCl) where sodium (Na) comes before the chlorine ion (Cl).
Ionic compounds exhibit a distinctive set of physical properties due to the strong electrostatic forces of attraction between the oppositely charged ions in their lattice structure. Below is a table that describes these properties in detail:
Property | Description |
|---|---|
High Melting and Boiling Points | Ionic compounds typically have high melting and boiling points. This is due to the strong ionic bonds that require significant amounts of energy to break. |
Hardness and Brittleness | Ionic compounds are generally hard because of the strong binding forces between the ions. However, they are also brittle and can shatter when subjected to a force. |
Electrical Conductivity | In the solid state, ionic compounds do not conduct electricity because the ions are fixed in place. However, when melted or dissolved in water, they conduct electricity due to the mobility of ions. |
Solubility in Water | Many ionic compounds are soluble in water. The polarity of water molecules enables them to surround and separate the ions, leading to dissolution. |
Density | Ionic compounds tend to have high densities due to the close packing of ions in their lattice structure. |
Cause: Strong electrostatic forces between ions.
Effect: Significant energy required to break these bonds.
Cause: Strong binding forces in a rigid lattice structure.
Effect: Compounds are hard but will shatter under stress as the lattice fractures.
State-dependent:
Solid state: Ions fixed in place, no conductivity.
Liquid state or aqueous solution: Ions free to move, allowing for conductivity.
Mechanism: Polar water molecules can hydrate and separate the ions.
Variability: Depends on the strength of the ionic bonds and the ion’s attraction to water molecules.
Cause: Close-packed ion arrangement in the lattice.
Implication: Results in a higher density compared to many other types of substances.
Ionic compounds are chemical compounds made up of ions held together by ionic bonds. These ions are atoms or molecules that have gained or lost electrons, resulting in a net charge. The formation of ionic compounds is primarily between metals and non-metals. Here are some key characteristics of ionic compounds:
Ionic compounds form a crystalline lattice structure. This regular, repeating pattern of ions results in the formation of solid crystals at room temperature. The structure is highly organized, allowing ionic compounds to have high melting and boiling points.
Due to the strong electrostatic forces of attraction between the oppositely charged ions, ionic compounds typically have high melting and boiling points. This means they are solid at room temperature and require a significant amount of energy to change into a liquid or gas.
In solid form, ionic compounds do not conduct electricity because the ions are fixed in place within the crystal lattice and cannot move freely. However, when dissolved in water or melted into a liquid, ionic compounds conduct electricity. This is because the ions are free to move and can carry charge through the solution or molten liquid.
Many ionic compounds are soluble in water. The polarity of water molecules allows them to interact with the positive and negative ions in an ionic compound, facilitating the compound’s dissolution. The extent of solubility varies among different ionic compounds, depending on the strength of the ionic bonds and the specific ions involved.
Despite their strength, ionic compounds are brittle. When force is applied, the crystal lattice can be distorted, causing ions of the same charge to be brought closer together. This repulsion between like-charged ions can cause the crystal to shatter or break apart.
Ionic compounds typically form when metals react with non-metals. Metals tend to lose electrons to achieve a stable electron configuration, becoming positively charged ions (cations). Non-metals tend to gain these electrons, becoming negatively charged ions (anions). The electrostatic attraction between these oppositely charged ions leads to the formation of ionic compounds.
The formation of ionic compounds is usually exothermic, meaning it releases energy. This release of energy occurs because the formation of strong ionic bonds between the ions releases more energy than is required to separate the electrons from the atoms.
One of the biggest properties of an ionic compound is its ability to conduct electricity in various states of matter. This means that if the ionic compound is in an aqueous form or is in a molten state, then it will be able to easily and effectively conduct electricity through its body. This quality exists in aqueous and molten ionic compounds because of the free-flow movement of all the ions in the compound. But if the ionic compound is in a solid state then it cannot conduct electricity due to the static position of all the ions in the compound.
Ionic compounds are very stable and will require great effort to split the atoms apart through dissolution. The ionic bond of the atoms in the compound causes the neutralization of all the charges of the compounds, which creates a very stable bond. Examples of ionic compounds used in our everyday life are table salt (sodium chloride or NaCl), baking soda (Na?2HCO^3 or sodium bicarbonate), and Calcium nitride (Ca3N2). Most of these ionic compounds are stable and will not immediately separate or create a new mixture upon contact with air and water.
Yes, ionic compounds can generally become soluble when immersed in a polar substance. This is due to their ionic bonds and their neutral charges. But not all ionic compounds are safe to mix with water. Calcium nitride has a reaction with water that creates an invisible flammable gas that can irritate and corrode one’s lungs, which will inadvertently lead to lung disease or damage. Not only that, but calcium nitride also creates a gas that can spontaneously combust, after reacting with water molecules in the atmosphere. This means that most ionic compounds are water-soluble, but not all can be safely mixed with water.
Ionic compounds are a type of mixture and compound that people create when they mix two or more atoms or substances that will end up with an ionic bond. These types of compounds have their own nomenclature. Future scientists and chemists should understand and know the importance of ionic compounds and how to create and name said compounds in a lab.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary force that holds ionic compounds together?
Covalent bonds
Ionic bonds
Metallic bonds
Hydrogen bonds
Which property is most common in ionic compounds at room temperature?
Gaseous state
Liquid state
Solid state
Plasma state
Ionic compounds conduct electricity when they are in which state?
Solid
Dissolved in water
Both A and B
Neither A nor B
What is the formula unit for sodium chloride?
NaCl
Na2Cl
NaCl2
Na2Cl2
Which is not a characteristic of ionic compounds?
High melting points
Low boiling points
Brittle
Soluble in water
What type of elements usually form ionic compounds?
Nonmetals only
Metals only
Metals and nonmetals
Metalloids and metals
What happens to the electron configuration of an atom in an ionic bond?
Electrons are shared evenly
Electrons are completely transferred
Electrons are shared unevenly
Electrons are unaffected
Which of the following is not likely to form an ionic bond?
Sodium (Na)
Chlorine (Cl)
Oxygen (O)
Carbon (C)
An ionic compound formed between magnesium and oxygen would be named:
Magnesium oxygen
Magnesium oxide
Magnesium oxy
Magnesium peroxide
Why do ionic compounds have high melting points?
Weak intermolecular forces
Strong covalent bonds
Strong ionic bonds
High molecular weights
Before you leave, take our quick quiz to enhance your learning!

