What is the primary use of iron sulfate in agriculture?
To increase soil acidity
To improve soil drainage
To treat iron deficiency in plants
To prevent soil erosion


Iron sulfate is a chemistry compound that combines iron and sulfur to form a salt. It appears as a blue-green crystalline solid and is commonly used in various applications, such as in fertilizers to supply essential nutrients to plants. Iron sulfate is also used in water treatment processes to remove impurities. This compound plays a vital role in the production of iron pigments and can be found in some medical treatments for iron deficiency. Its versatility makes it an important substance in both industrial and agricultural chemistry.
| Property | Value |
|---|---|
| Formula | FeSO4·7H2O |
| Hill Formula | FeH14O11S |
| Name | Ironate |
| IUPAC Name | Iron(+2) Cation Sulfate Heptahydrate |
| Alternate Names | Ferrous Sulfate, Ferrous Sulfate Heptahydrate, Haemofort, Iron(II) Sulfate |
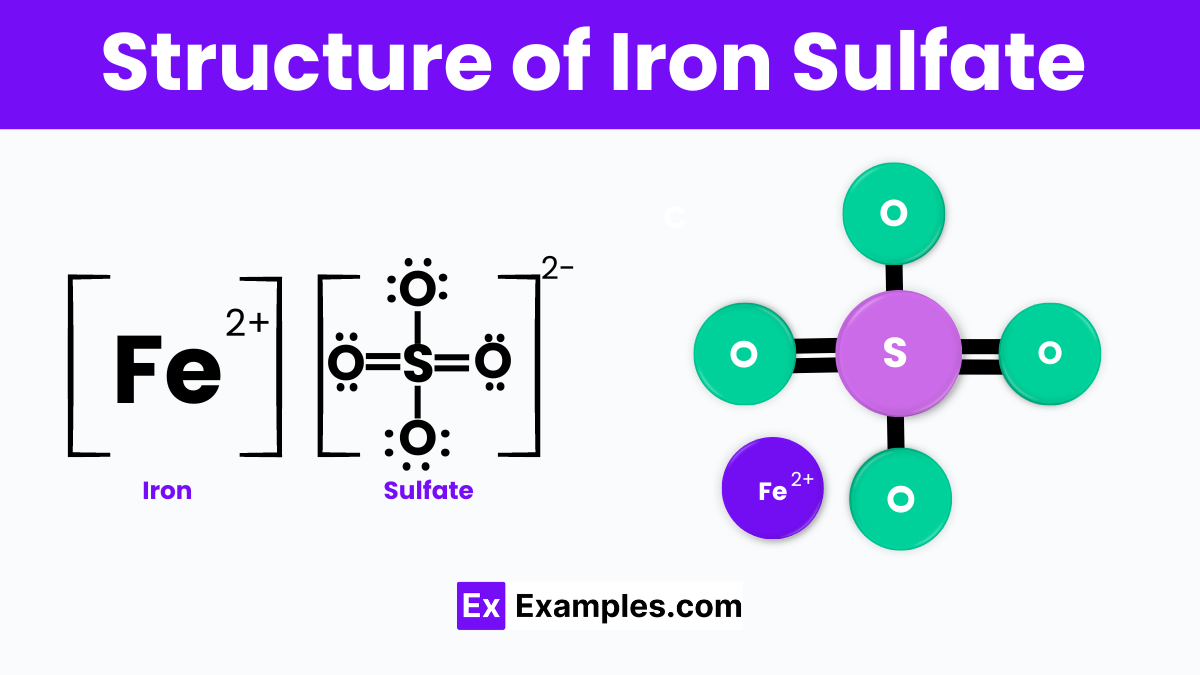
The structure of iron sulfate consists of one iron ion (Fe²⁺) and one sulfate ion (SO₄²⁻). In this compound, the iron ion is positively charged, while the sulfate ion is negatively charged, allowing them to bond together through ionic bonds. The sulfate ion has a tetrahedral shape, where one sulfur atom is centrally located and surrounded by four oxygen atoms. These ions form a crystalline structure when solid, often appearing as blue-green crystals.
To prepare iron sulfate, you can react iron with sulfuric acid. This process involves placing a piece of iron into a container with diluted sulfuric acid. As the reaction occurs, iron dissolves and forms iron sulfate and hydrogen gas. You can observe bubbles of hydrogen gas forming as the reaction proceeds. The chemical equation for this reaction is:
After the reaction completes, you can evaporate the remaining liquid to obtain solid iron sulfate crystals. This method is commonly used in laboratories to produce iron sulfate for various experiments and applications.
| Property | Description |
|---|---|
| Appearance | Iron sulfate appears as blue-green crystals. |
| Solubility | It dissolves easily in water. |
| Melting Point | Iron sulfate has a melting point of 64°C (147°F). |
| Density | The density of iron sulfate is about 1.898 g/cm³. |
| Odor | It does not have a significant odor. |
| Molecular Weight | The molecular weight of iron sulfate is 151.91 g/mol. |
Iron sulfate dissolves in water, forming a pale green solution. This solution is often used for various industrial and medical applications.
FeSO₄ → Fe²⁺ +SO₄²⁻
When exposed to air, iron sulfate oxidizes to form iron(III) sulfate and a brownish-yellow coating of iron(III) oxide. This reaction shows the change in the oxidation state of iron from +2 to +3.
4FeSO₄ + O₂ + 2H₂O → 2Fe₂(SO₄)₃ + 2H₂O
Iron sulfate reacts with alkalis, such as sodium hydroxide, to form iron(II) hydroxide, which precipitates as a green solid.
FeSO₄ + 2NaOH → Fe(OH)₂ + Na₂SO₄
When heated, iron sulfate decomposes to form iron(III) oxide, sulfur dioxide, and sulfur trioxide. This reaction releases gases and changes the chemical composition of iron sulfate.
2FeSO₄ → Fe₂O₃ + SO₂ + SO₃
| Property | Value |
|---|---|
| CAS Registry Number | 7782-63-0 |
| PubChem Compound ID | 62662 |
| SMILES Identifier | O.O.O.O.O.O.O.[O-]S(=O)(=O)[O-].[Fe+2] |
| InChI Identifier | InChI=1/Fe.H2O4S.7H2O/c;1-5(2,3)4;;;;;;;/h;(H2,1,2,3,4);7*1H2/q+2;;;;;;;;/p-2/fFe.O4S.7H2O/qm;-2;;;;;;; |
| EU Number | 231-753-5 |
| Gmelin Number | 105946 |
| RTECS Number | NO8510000 |
| Property | Value |
|---|---|
| NFPA Health Rating | 1 |
| NFPA Fire Rating | 0 |
| NFPA Reactivity Rating | 0 |
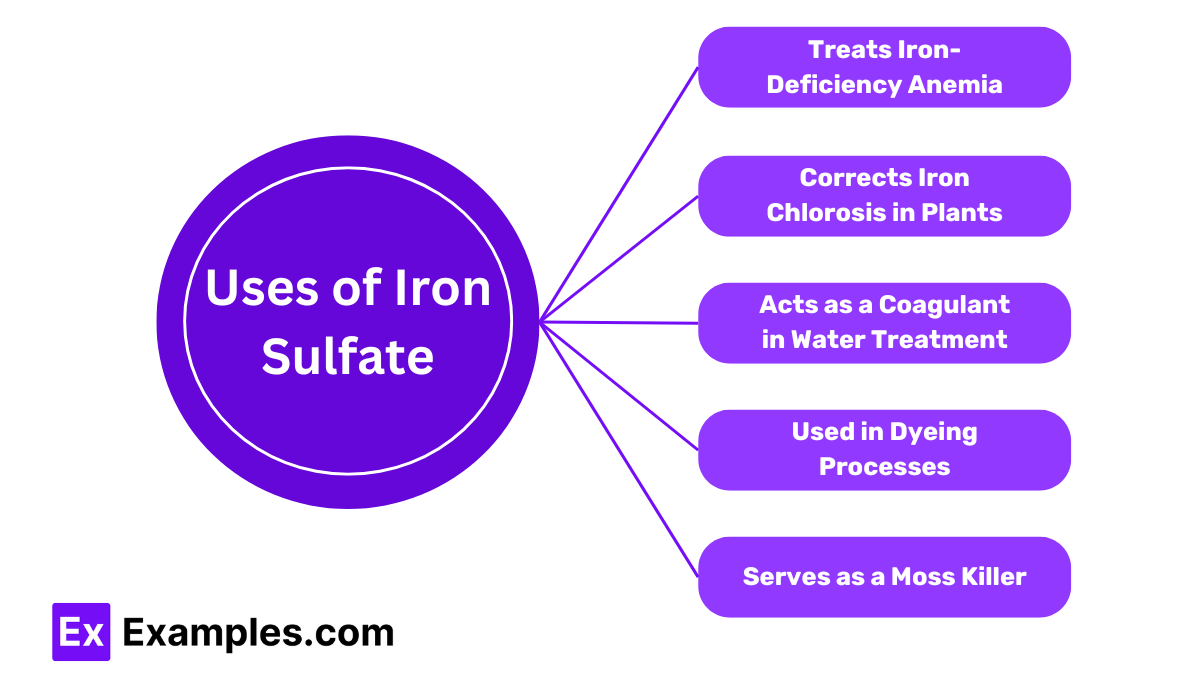
Doctors prescribe iron sulfate to treat iron-deficiency anemia. It provides the necessary iron for the body to produce red blood cells.
Gardeners use iron sulfate to correct iron chlorosis in plants. It helps plants develop healthy green leaves by supplying essential iron.
Iron sulfate acts as a coagulant in water treatment plants. It helps remove impurities from water by clumping particles together for easier filtration.
The textile industry uses iron sulfate in dyeing processes. It helps fix dyes to fabrics, ensuring vibrant and long-lasting colors.
Iron sulfate is effective as a moss killer on lawns. It helps eliminate moss, promoting healthier grass growth.
Chemists use iron sulfate to produce other iron compounds. It serves as a starting material for synthesizing various chemicals in laboratories.
Take ferrous sulfate on an empty stomach, preferably one hour before or two hours after meals, to enhance absorption. If stomach upset occurs, take it with food.
Ferrous sulfate is added to food as a fortificant to prevent iron deficiency, ensuring adequate iron intake, especially in populations at risk for anemia.
Ferrous sulfate is an iron supplement used to treat or prevent iron-deficiency anemia by providing the body with essential iron needed for red blood cell production.
Avoid dairy products, coffee, tea, eggs, and high-fiber foods when taking iron sulfate, as they can interfere with iron absorption.
Adults typically take 100-200 mg of elemental iron daily, divided into two or three doses. Always follow your healthcare provider’s recommendations for dosage.
Iron sulfate acts as a coagulant, helping to remove impurities from water by clumping particles together for easier filtration.
Iron sulfate corrects iron chlorosis in plants, promoting healthy green leaves and overall plant health by providing essential iron.
Iron sulfate is safe when used as directed but can cause side effects. Consult a healthcare provider for medical use and follow guidelines for gardening or industrial applications.
Ferrous sulfate liquid can stain teeth. To prevent this, mix the liquid with water or juice, drink through a straw, and brush teeth afterwards.
Yes, taking ferrous sulfate with vitamin C can enhance iron absorption. Vitamin C helps convert iron into a form that is easier for the body to absorb.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary use of iron sulfate in agriculture?
To increase soil acidity
To improve soil drainage
To treat iron deficiency in plants
To prevent soil erosion
What is the chemical formula for iron sulfate?
FeSO₄
Fe₂O₃
FeCl₃
Fe(NO₃)₂
In which of the following conditions is iron sulfate commonly used in medicine?
To treat high blood pressure
To treat iron deficiency anemia
To reduce cholesterol levels
To manage diabetes
How is iron sulfate typically administered in medical treatments?
As an injection
In tablet or liquid form
As a topical ointment
As a dietary supplement
Which form of iron sulfate is used for soil amendment?
Ferrous sulfate
Iron chloride
Ferric sulfate
Iron oxide
What is a common side effect of taking iron sulfate supplements?
Nausea
Insomnia
Dry skin
Headaches
Why is iron sulfate used in the water treatment process?
To remove impurities
To enhance water flavor
To add essential nutrients
To disinfect water
What is the appearance of iron sulfate crystals?
White powder
Red-brown crystals
Greenish-blue crystals
Blue powder
Which industry frequently utilizes iron sulfate as a coagulant?
Textile
Paper manufacturing
Construction
Electronics
How should iron sulfate be stored to ensure its effectiveness?
In a humid environment
In a well-sealed container away from light
In an open container
In a refrigerator
Before you leave, take our quick quiz to enhance your learning!

