What is the chemical formula of Iron(II) Sulfide?
FeS
Fe2S3
FeSO4
FeS2

Iron(II) sulfide is a fascinating chemical compound that plays a significant role in various fields, including geology, chemistry, and industry. Made up of iron and sulfur, this compound is known for its dark, metallic appearance and is commonly found in the natural environment, especially within the earth’s crust. Iron(II) sulfide is formed when iron and sulfur combine in a specific ratio, creating a substance that is not only interesting for scientists to study but also has practical applications in everyday life. For example, it’s used in the production of sulfuric acid, in the iron and steel industry, and even in the preparation of certain pigments. Despite its simplicity, iron(II) sulfide’s unique properties make it an important material for various scientific and industrial processes.
| Formula | FeS |
| Name | Ferrous sulfide |
| Alternate Names | Ferrous sulfide (Iron(II) sulfide), Iron(II) sulfide, Iron monosulfide, Sulfanyliden iron, Thioxoiron |
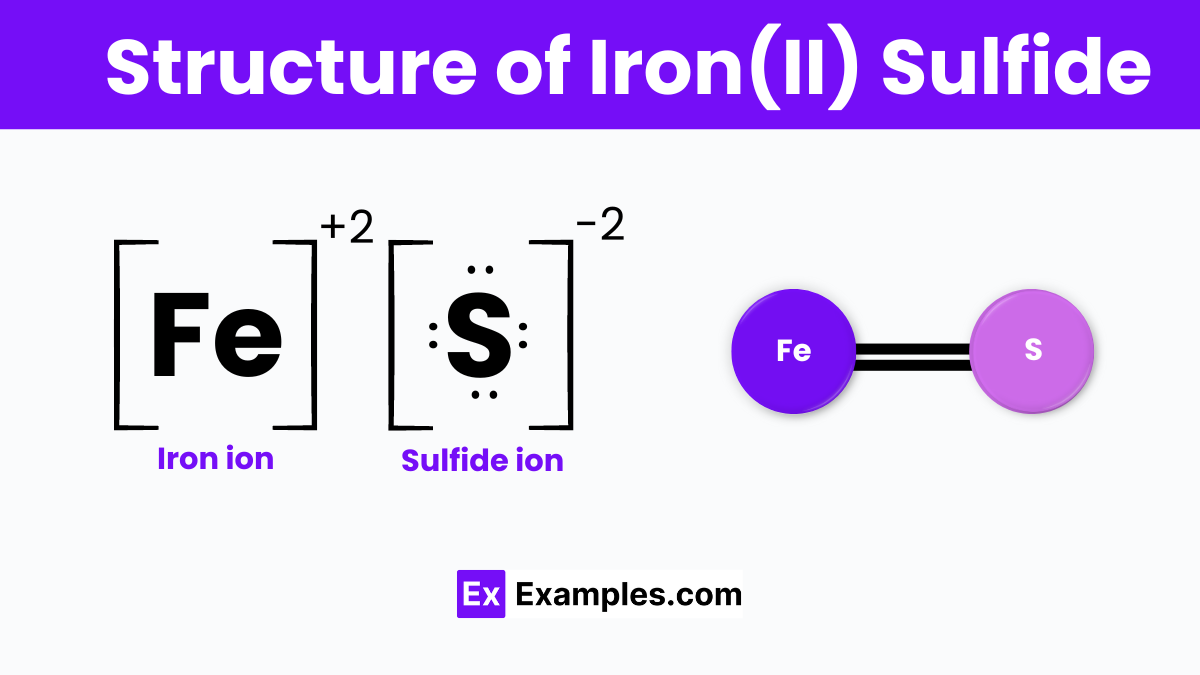
Iron(II) sulfide, or ferrous sulfide, is a compound formed by iron and sulfur in a 1:1 ratio, creating a crystal lattice where these atoms alternate. This structure is reminiscent of a grid made up of tiny balls, with each iron atom closely surrounded by sulfur atoms, facilitating a solid bond. This arrangement results in a solid material often found as the mineral pyrrhotite, characterized by a metallic luster and magnetic properties.
The crystalline form of iron(II) sulfide features a layered structure that contributes to its unique characteristics, such as its brittle nature and limited electrical conductivity, differentiating it from pure metals. FeS is not only crucial in various industrial applications but also plays a significant role in the iron-sulfur world theory, suggesting a possible pathway for the origin of life on Earth through simple reactions involving iron and sulfur compounds.
Iron(II) sulfide, or FeS, is made by directly combining iron and sulfur. To do this safely in a lab, mix 7 grams of iron powder with 4 grams of sulfur in a porcelain crucible. This mixture is based on the chemical reaction ensuring the right balance between the iron and sulfur.
Once mixed, the crucible is heated slowly at first to prevent the mixture from splattering. As the heat increases, the sulfur melts, coats the iron, and eventually, the mix glows brightly, indicating the reaction has occurred. After cooling, the result is iron(II) sulfide, a metallic solid that doesn’t dissolve in water. This process requires careful handling, including wearing gloves and goggles, and working in a well-ventilated area to avoid inhaling toxic gases.
The preparation of iron(II) sulfide demonstrates an important chemical principle: the reaction between iron and sulfur is exothermic, releasing heat. This reaction is not only a fascinating example of how two elements combine to form a new compound but also shows the practical application of stoichiometry, the part of chemistry that studies the amounts of substances that react and form in chemical reactions. Iron(II) sulfide has various uses, including in the synthesis of other chemicals, in the iron and steel industries, and for educational demonstrations in chemistry classes. Its preparation, while simple, offers a clear window into the world of chemical synthesis and the careful balance and precautions necessary in any chemical experiment.
| Property | Description |
|---|---|
| Appearance | Dark, gray to black solid |
| Molecular Formula | FeS |
| Molar Mass | 87.91 g/mol |
| Density | 4.84 g/cm³ |
| Melting Point | 1195°C (2183°F) |
| Boiling Point | Decomposes before boiling |
| Solubility | Insoluble in water; slightly soluble in acid |
| Hardness | Brittle, with a Mohs hardness of 4-4.5 |
| Electrical Conductivity | Conductive, due to metallic bonding |
| Magnetic Properties | Ferromagnetic at temperatures below its Curie point |
| Thermal Stability | Stable under normal conditions but decomposes upon heating to sulfur dioxide and iron under high temperatures |
Iron(II) sulfide reacts with acids, forming hydrogen sulfide gas (H₂S) and an iron(II) salt. For example:
This demonstrates how FeS can generate hydrogen sulfide gas.
Exposed to oxygen, FeS oxidizes, forming iron(III) oxide and sulfur dioxide:
4FeS+7O₂→2Fe₂O₃+4SO₂
This reaction indicates FeS’s role in the sulfur cycle and its susceptibility to weathering.
FeS is insoluble in water. It doesn’t dissolve but can form minerals like pyrite in specific conditions.
FeS decomposes when heated in air, producing sulfur dioxide and iron(III) oxide:
4FeS+7O₂ →2Fe₂O₃+4SO₂
This occurs around 600°C, useful in metal extraction.
FeS is weakly magnetic. This allows magnetic separation from non-magnetic substances, aiding in mineral processing.
FeS’s oxidation releases sulfur dioxide and iron(III) compounds. This can lead to acid mine drainage, affecting water bodies and aquatic life negatively.
| Property | Value |
|---|---|
| CAS Registry Number | 1317-37-9 |
| PubChem Compound ID | 14828 |
| SMILES Identifier | [Fe]=S |
| MDL Number | MFCD00011013 |
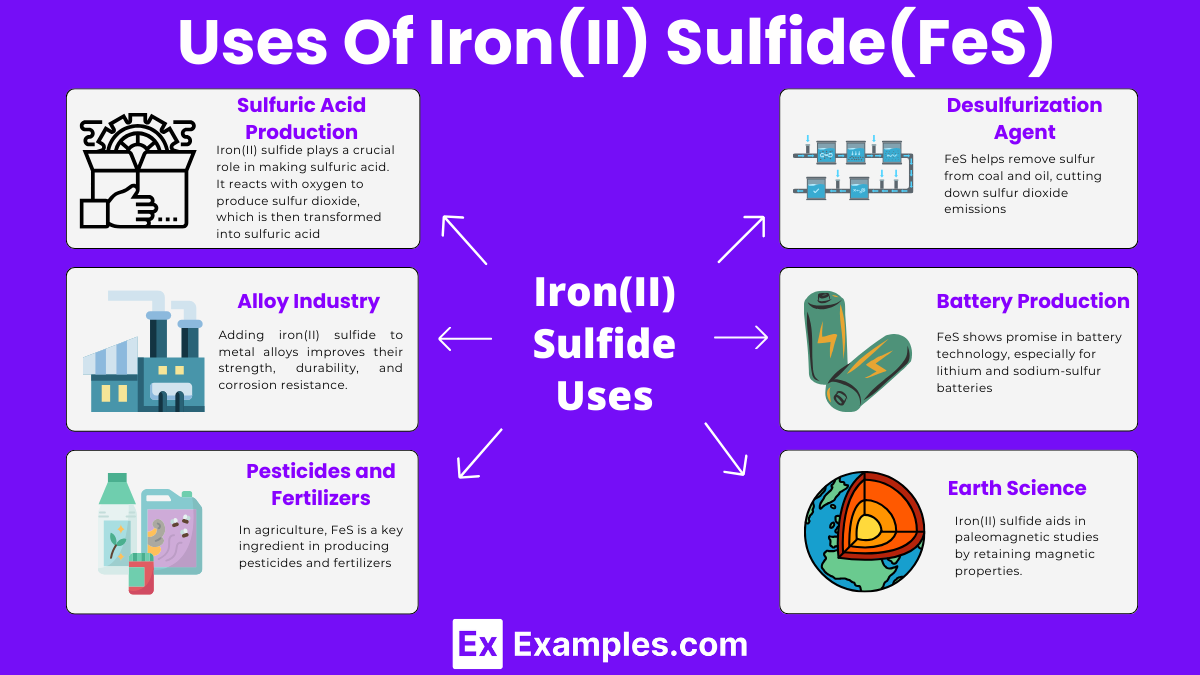
Iron(II) sulfide plays a crucial role in making sulfuric acid. It reacts with oxygen to produce sulfur dioxide, which is then transformed into sulfuric acid. This acid is essential for making fertilizers, in petroleum processing, and chemical manufacturing.
FeS helps remove sulfur from coal and oil, cutting down sulfur dioxide emissions. This process is vital for reducing air pollution and combating acid rain.
Adding iron(II) sulfide to metal alloys improves their strength, durability, and corrosion resistance. This enhancement is critical for materials used in construction, automotive, and aerospace sectors.
In agriculture, FeS is a key ingredient in producing pesticides and fertilizers. It supplies essential nutrients to plants and helps manage crop-damaging pests, boosting agricultural productivity.
FeS shows promise in battery technology, especially for lithium and sodium-sulfur batteries. Its conductive properties could lead to more efficient, durable, and eco-friendly batteries.
Iron(II) sulfide aids in paleomagnetic studies by retaining magnetic properties. These properties offer insights into the Earth’s past magnetic fields and geological activities.
Yes, FeS2 is a sulfide known as iron disulfide, commonly found as the mineral pyrite.
The correct name for FeS2 is iron disulfide, often referred to as pyrite or fool’s gold.
FeS, with one sulfur atom, is iron(II) sulfide because iron has a +2 oxidation state, forming a 1:1 ratio with sulfur.
FeS2, or iron disulfide, has covalent characteristics due to the sharing of electrons between iron and sulfur atoms.

Iron(II) sulfide is a fascinating chemical compound that plays a significant role in various fields, including geology, chemistry, and industry. Made up of iron and sulfur, this compound is known for its dark, metallic appearance and is commonly found in the natural environment, especially within the earth’s crust. Iron(II) sulfide is formed when iron and sulfur combine in a specific ratio, creating a substance that is not only interesting for scientists to study but also has practical applications in everyday life. For example, it’s used in the production of sulfuric acid, in the iron and steel industry, and even in the preparation of certain pigments. Despite its simplicity, iron(II) sulfide’s unique properties make it an important material for various scientific and industrial processes.
Iron(II) sulfide, represented chemically as FeS, is a distinct compound where iron and sulfur atoms bond in a one-to-one ratio. This compound is integral to the field of chemistry, showcasing the fascinating ways in which different elements can combine to form materials with unique properties. In its pure form, FeS exhibits a crystalline structure, showcasing the beauty and complexity of chemical reactions. Beyond its scientific intrigue, iron(II) sulfide has practical relevance in various applications, from environmental science to the manufacturing sector, underscoring its importance beyond the laboratory. It’s a prime example of how chemistry connects fundamental scientific principles with real-world uses, illustrating the transformative power of chemical compounds
Formula | FeS |
Name | Ferrous sulfide |
Alternate Names | Ferrous sulfide (Iron(II) sulfide), Iron(II) sulfide, Iron monosulfide, Sulfanyliden iron, Thioxoiron |

Iron(II) sulfide, or ferrous sulfide, is a compound formed by iron and sulfur in a 1:1 ratio, creating a crystal lattice where these atoms alternate. This structure is reminiscent of a grid made up of tiny balls, with each iron atom closely surrounded by sulfur atoms, facilitating a solid bond. This arrangement results in a solid material often found as the mineral pyrrhotite, characterized by a metallic luster and magnetic properties.
The crystalline form of iron(II) sulfide features a layered structure that contributes to its unique characteristics, such as its brittle nature and limited electrical conductivity, differentiating it from pure metals. FeS is not only crucial in various industrial applications but also plays a significant role in the iron-sulfur world theory, suggesting a possible pathway for the origin of life on Earth through simple reactions involving iron and sulfur compounds.
Iron(II) sulfide, or FeS, is made by directly combining iron and sulfur. To do this safely in a lab, mix 7 grams of iron powder with 4 grams of sulfur in a porcelain crucible. This mixture is based on the chemical reaction ensuring the right balance between the iron and sulfur.
Fe + S → FeS
Once mixed, the crucible is heated slowly at first to prevent the mixture from splattering. As the heat increases, the sulfur melts, coats the iron, and eventually, the mix glows brightly, indicating the reaction has occurred. After cooling, the result is iron(II) sulfide, a metallic solid that doesn’t dissolve in water. This process requires careful handling, including wearing gloves and goggles, and working in a well-ventilated area to avoid inhaling toxic gases.
The preparation of iron(II) sulfide demonstrates an important chemical principle: the reaction between iron and sulfur is exothermic, releasing heat. This reaction is not only a fascinating example of how two elements combine to form a new compound but also shows the practical application of stoichiometry, the part of chemistry that studies the amounts of substances that react and form in chemical reactions. Iron(II) sulfide has various uses, including in the synthesis of other chemicals, in the iron and steel industries, and for educational demonstrations in chemistry classes. Its preparation, while simple, offers a clear window into the world of chemical synthesis and the careful balance and precautions necessary in any chemical experiment.
Property | Description |
|---|---|
Appearance | Dark, gray to black solid |
Molecular Formula | FeS |
Molar Mass | 87.91 g/mol |
Density | 4.84 g/cm³ |
Melting Point | 1195°C (2183°F) |
Boiling Point | Decomposes before boiling |
Solubility | Insoluble in water; slightly soluble in acid |
Hardness | Brittle, with a Mohs hardness of 4-4.5 |
Electrical Conductivity | Conductive, due to metallic bonding |
Magnetic Properties | Ferromagnetic at temperatures below its Curie point |
Thermal Stability | Stable under normal conditions but decomposes upon heating to sulfur dioxide and iron under high temperatures |
Iron(II) sulfide reacts with acids, forming hydrogen sulfide gas (H₂S) and an iron(II) salt. For example:
Reaction with Hydrochloric Acid : FeS+2HCl→FeCl+H₂S
This demonstrates how FeS can generate hydrogen sulfide gas.
Exposed to oxygen, FeS oxidizes, forming iron(III) oxide and sulfur dioxide:
4FeS+7O₂→2Fe₂O₃+4SO₂
This reaction indicates FeS’s role in the sulfur cycle and its susceptibility to weathering.
FeS is insoluble in water. It doesn’t dissolve but can form minerals like pyrite in specific conditions.
FeS decomposes when heated in air, producing sulfur dioxide and iron(III) oxide:
4FeS+7O₂ →2Fe₂O₃+4SO₂
This occurs around 600°C, useful in metal extraction.
FeS is weakly magnetic. This allows magnetic separation from non-magnetic substances, aiding in mineral processing.
FeS’s oxidation releases sulfur dioxide and iron(III) compounds. This can lead to acid mine drainage, affecting water bodies and aquatic life negatively.
Property | Value |
|---|---|
CAS Registry Number | 1317-37-9 |
PubChem Compound ID | 14828 |
SMILES Identifier | [Fe]=S |
MDL Number | MFCD00011013 |

Iron(II) sulfide plays a crucial role in making sulfuric acid. It reacts with oxygen to produce sulfur dioxide, which is then transformed into sulfuric acid. This acid is essential for making fertilizers, in petroleum processing, and chemical manufacturing.
FeS helps remove sulfur from coal and oil, cutting down sulfur dioxide emissions. This process is vital for reducing air pollution and combating acid rain.
Adding iron(II) sulfide to metal alloys improves their strength, durability, and corrosion resistance. This enhancement is critical for materials used in construction, automotive, and aerospace sectors.
In agriculture, FeS is a key ingredient in producing pesticides and fertilizers. It supplies essential nutrients to plants and helps manage crop-damaging pests, boosting agricultural productivity.
FeS shows promise in battery technology, especially for lithium and sodium-sulfur batteries. Its conductive properties could lead to more efficient, durable, and eco-friendly batteries.
Iron(II) sulfide aids in paleomagnetic studies by retaining magnetic properties. These properties offer insights into the Earth’s past magnetic fields and geological activities.
Metal Processing: FeS helps extract metals and make sulfuric acid.
Catalysis: It speeds up chemical production, improving process efficiency.
Heavy Metal Removal: FeS cleans water by removing heavy metals.
Nutrient Release: It plays a role in the sulfur cycle, enriching soils and aiding plant growth.
Hydrogen Sulfide Source: FeS is used to produce H2S for various industries.
Battery Technology: It’s being studied for use in batteries, promising better energy storage.
Fossil Preservation: FeS helps preserve fossils over millions of years.
Geological Insights: Its presence in rocks tells us about past environments.
Soil Improvement: Adding FeS can adjust soil pH, benefiting crop growth.
Lower Risk: FeS is safer to handle than many sulfur compounds, reducing hazards.
Acid Mine Drainage: When FeS is exposed to air and water, it can produce sulfuric acid, leading to acid mine drainage. This phenomenon can harm aquatic life and water quality.
Soil Acidification: FeS oxidation can also acidify soils, impacting plant growth and soil health.
Hydrogen Sulfide Gas: Reacting FeS with acids releases hydrogen sulfide (H2S), a toxic gas. H2S exposure can cause respiratory issues and, in high concentrations, can be fatal.
Dust Inhalation: Inhaling FeS dust can irritate the respiratory system, causing coughing and shortness of breath.
Fire Hazard: FeS dust is flammable and, when mixed with air, can form explosive mixtures.
Corrosion: FeS can accelerate the corrosion of metal structures, especially in humid environments, posing risks to infrastructure.
Safe Storage: FeS must be stored properly to prevent unwanted reactions with air or moisture.
Personal Protective Equipment (PPE): Proper PPE is necessary to handle FeS safely, including gloves, masks, and eye protection.
Yes, FeS2 is a sulfide known as iron disulfide, commonly found as the mineral pyrite.
The correct name for FeS2 is iron disulfide, often referred to as pyrite or fool’s gold.
FeS, with one sulfur atom, is iron(II) sulfide because iron has a +2 oxidation state, forming a 1:1 ratio with sulfur.
FeS2, or iron disulfide, has covalent characteristics due to the sharing of electrons between iron and sulfur atoms.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical formula of Iron(II) Sulfide?
FeS
Fe2S3
FeSO4
FeS2
What is the primary use of Iron Sulfide in industry?
Fertilizer production
Manufacture of sulfuric acid
Food preservation
Textile dyeing
Which type of bond is primarily found in Iron Sulfide?
Ionic bond
Covalent bond
Metallic bond
Hydrogen bond
What is the appearance of Iron(II) Sulfide in its pure form?
White crystalline solid
Yellow powder
Black or brown solid
Colorless liquid
Iron Sulfide can react with hydrochloric acid to produce which gas?
Oxygen
Nitrogen
Hydrogen
Hydrogen sulfide
Which mineral form of Iron Sulfide is known as fool's gold?
Pyrite
Hematite
Magnetite
Chalcopyrite
Iron Sulfide can be formed by direct combination of iron and sulfur. What type of reaction is this?
Decomposition
Single replacement
Synthesis
Double replacement
Which of the following is a common application of Iron(II) Sulfide in laboratories?
As a desiccant
In qualitative analysis
As a reducing agent
In electroplating
What is the solubility of Iron(II) Sulfide in water?
Highly soluble
Moderately soluble
Sparingly soluble
Insoluble
What is the common oxidation state of sulfur in Iron(II) Sulfide?
-2
-1
0
+1
Before you leave, take our quick quiz to enhance your learning!

