What is the atomic number of krypton?
34
36
38
40

Enter the noble world of Krypton, an element that’s not just a part of superhero lore but a real-life gas with unique properties. In this 75-word guide, teachers will find rich information and practical examples to bring the wonders of Krypton into the classroom. From its use in lighting to its role in scientific measurements, Krypton’s versatility is bound to spark curiosity and enhance learning. Let’s uncover the secrets of Krypton together, making science education more dynamic and engaging.
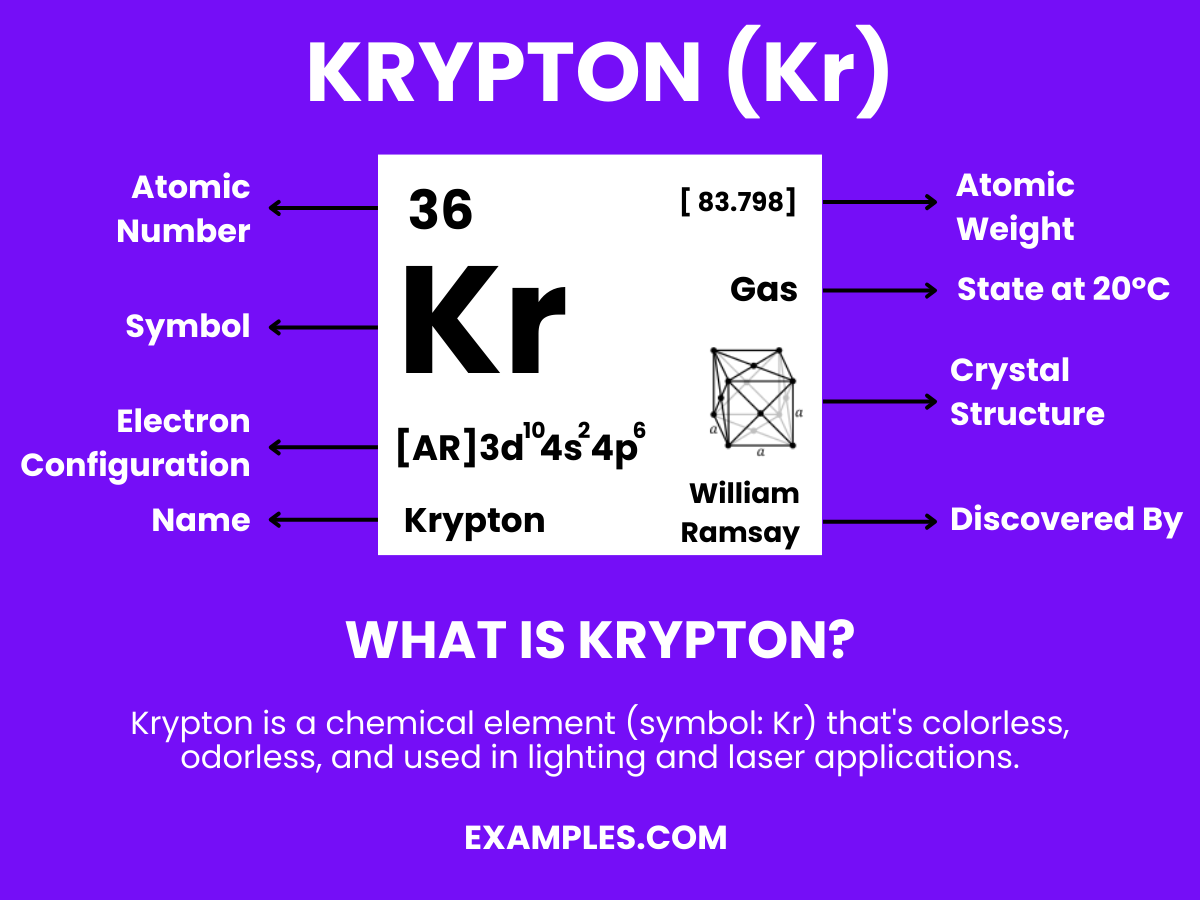
Krypton is a noble gas with the chemical symbol Kr and atomic number 36. It’s colorless, odorless, and tasteless, and is found in trace amounts in the Earth’s atmosphere. Krypton is known for its stability and inertness, rarely forming compounds. It’s used in various applications, including high-intensity lighting, fluorescent lamps, and as a standard in measuring the length (as the definition of a meter was once based on krypton’s spectral lines). Its rarity and unique properties make it a fascinating subject in physics and chemistry education.
| Helium |
| Neon |
| Argon |
| Xenon |
| Radon |
| Property | Description |
|---|---|
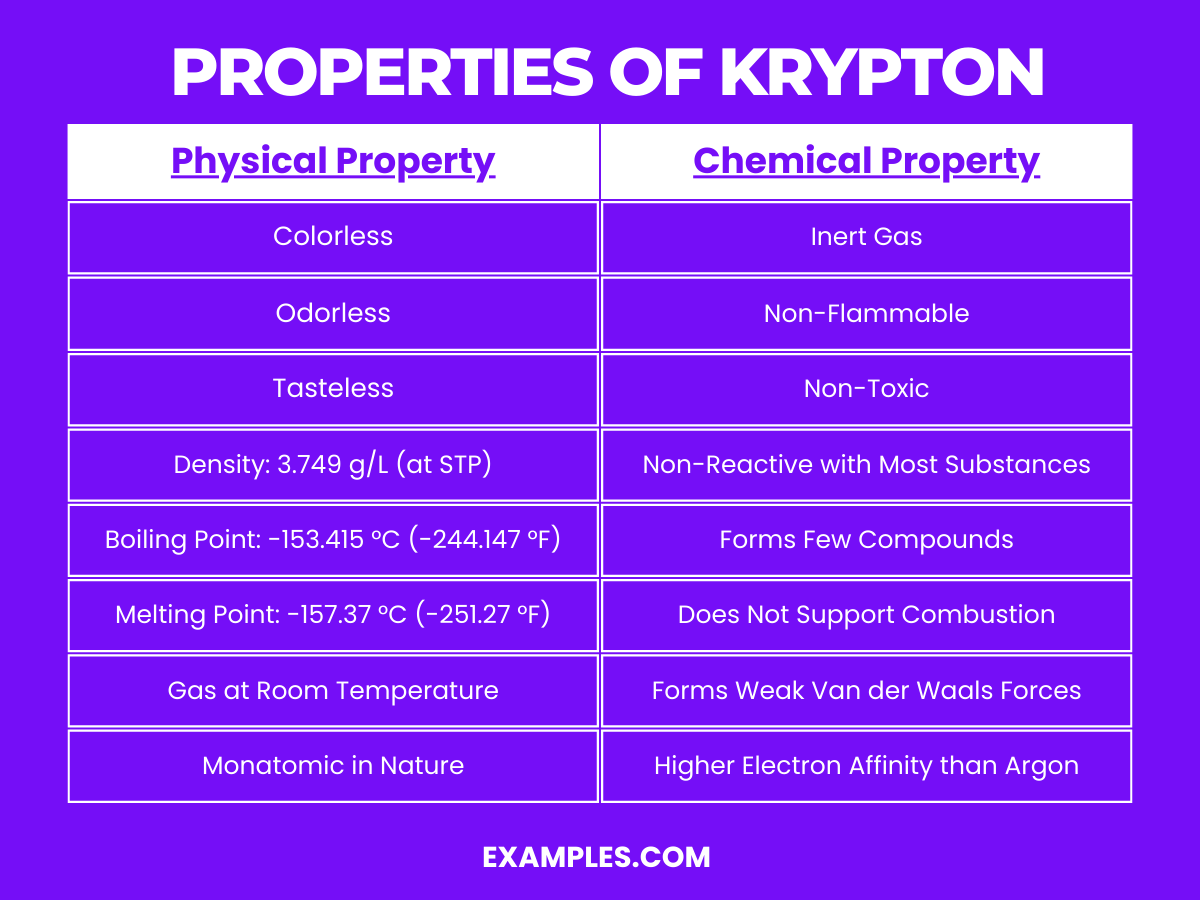
| State at Room Temperature | Gas |
| Color | Colorless |
| Odor | Odorless |
| Taste | Tasteless |
| Density | 3.749 g/L at 0°C and 1 atm |
| Boiling Point | -153.415 °C (-244.147 °F) |
| Melting Point | -157.37 °C (-251.27 °F) |
| Monatomic Nature | Exists as single atoms in gaseous state |
Krypton is another noble gas, exhibiting chemical properties typical to its group, primarily characterized by low reactivity due to its complete valence electron shell:
| Property | Description / Value |
|---|---|
| Melting Point | -157.36°C (-251.25°F) |
| Boiling Point | -153.22°C (-243.80°F) |
| Thermal Conductivity | 9.43 × 10^-3 W/(m·K) at 273K |
| Specific Heat | 0.248 J/(g·K) at 20°C |
| Heat of Vaporization | 9.08 kJ/mol at boiling point |
| Heat of Fusion | 1.64 kJ/mol at melting point |
| Property | Description / Value |
|---|---|
| Phase at STP | Gas |
| Density | 3.749 g/L at 0°C, 101.325 kPa (gas) |
| Approximately 2.413 g/cm³ at -153°C (liquid) | |
| Solubility in Water | 0.114 mg/L at 20°C |
| Color | Colorless |
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Diamagnetic |
| Electrical Conductivity | Non-conductor (Krypton is a dielectric material) |
| Property | Description / Value |
|---|---|
| Atomic Number | 36 |
| Atomic Mass | 83.798 u |
| Neutron Cross Section | 25 barns (for ^84Kr) |
| Isotopes | Stable: ^78Kr, ^80Kr, ^82Kr, ^83Kr, ^84Kr, ^86Kr |
| Radioactive: ^81Kr, ^85Kr (and others with shorter half-lives) | |
| Radioactivity | ^85Kr (half-life: 10.76 years), ^81Kr (used in dating with a half-life of 229,000 years) |
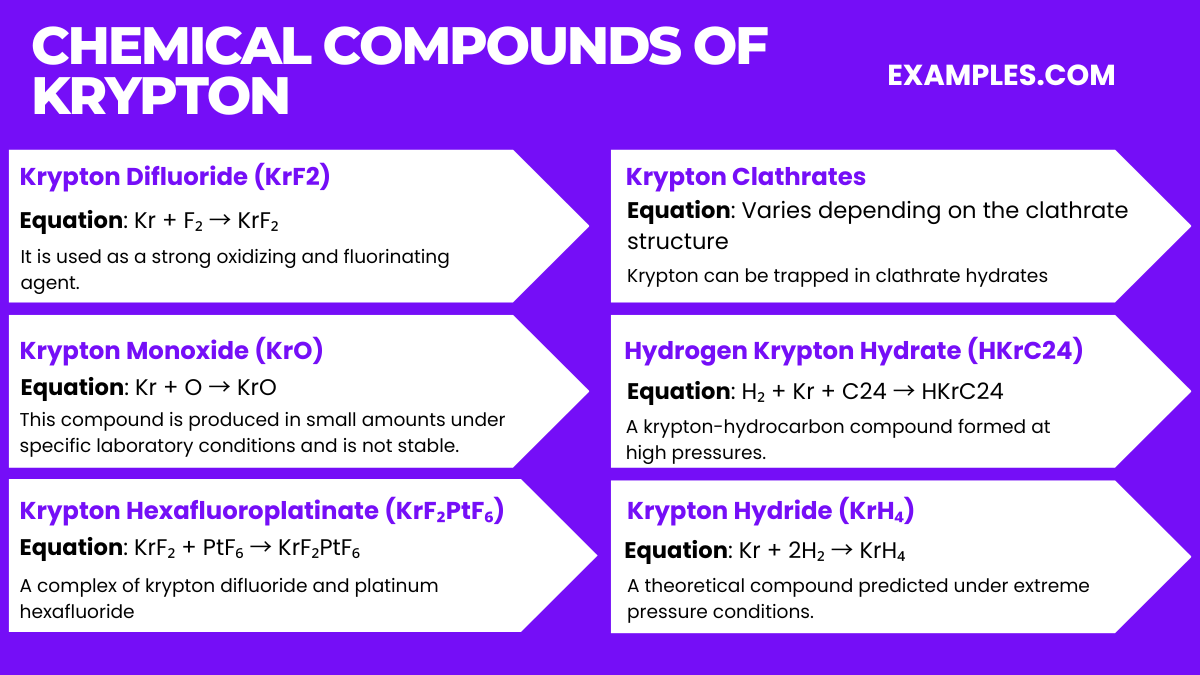
Krypton, being a noble gas, forms very few compounds, and they are generally unstable. However, here are some compounds that have been identified:
| Isotope | Natural Abundance | Atomic Mass | Half-Life | Description |
|---|---|---|---|---|
| Krypton-78 | Trace | 77.9204 u | Stable | Trace isotope found in the atmosphere. |
| Krypton-80 | 2.28% | 79.9164 u | Stable | One of the stable isotopes, used in the study of atmospheric krypton. |
| Krypton-82 | 11.58% | 81.9135 u | Stable | The most abundant stable isotope of krypton. |
| Krypton-83 | 11.49% | 82.9141 u | Stable | Used in magnetic resonance imaging (MRI) for imaging airways and lungs. |
| Krypton-84 | 57% | 83.9115 u | Stable | The second most abundant stable isotope of krypton. |
| Krypton-85 | Trace (radioactive) | 84.9125 u | 10.756 years | A byproduct of nuclear fission with applications in leak detection and atmospheric studies. |
| Krypton-86 | 17.3% | 85.9106 u | Stable | Used to define the standard meter from 1960 to 1983, as the wavelength of light from krypton-86 isotope was used. |
Krypton is commercially produced through the fractional distillation of liquid air. This process involves the cooling of air to a point where it liquefies, followed by gradual warming that allows the separate gases to boil off at their respective boiling points. Here are the key steps:
Krypton is a noble gas that is generally non-toxic and chemically inert. However, it can have specific health effects under certain conditions:
Safety measures, such as adequate ventilation and the use of appropriate protective equipment, are crucial when working with krypton in industrial or laboratory settings to mitigate the risks associated with its physical properties.
Krypton is a naturally occurring element in the Earth’s atmosphere and is present in trace amounts. Here are its environmental effects:
Krypton is largely non-toxic but can cause asphyxiation by displacing oxygen in enclosed spaces, leading to dizziness or unconsciousness.
Krypton is cool due to its rare, inert nature, brilliant light emission in bulbs, and unique applications in high-tech fields like laser technology and photography.
Krypton is called a hidden gas due to its rare and inert nature, derived from the Greek word “kryptos,” meaning hidden, reflecting its elusive presence.
Krypton is used in lighting (fluorescent bulbs), insulation for windows, high-speed photography, and MRI lung imaging, capitalizing on its inert properties.
Krypton is a noble gas with unique applications, from lighting to high-speed photography. Its inertness ensures safety and effectiveness in various industries. Understanding Krypton’s properties, safe handling, and innovative uses is essential for its optimal application. Stay informed and cautious to utilize Krypton’s benefits efficiently and safely in your endeavors.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
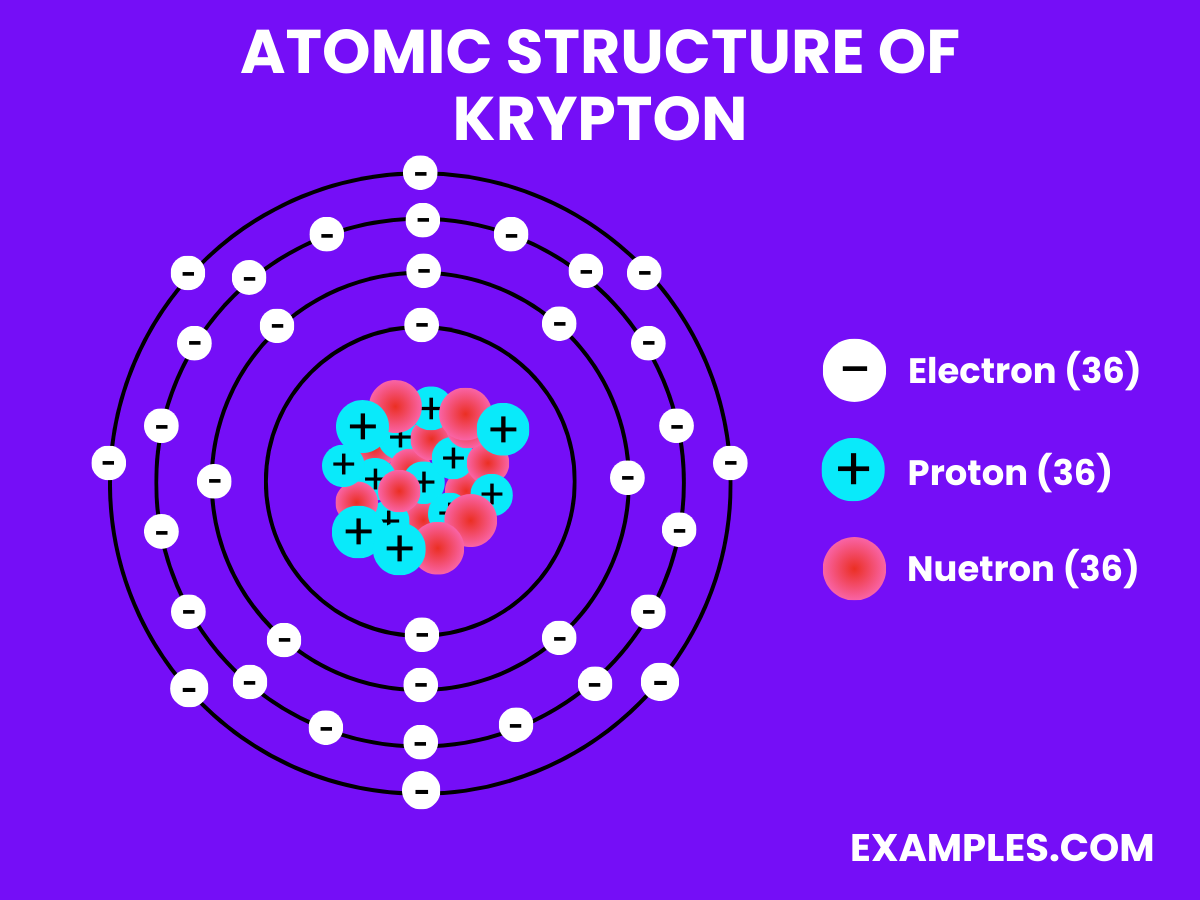
Electrons
Neutrons
Protons
What is the atomic number of krypton?
34
36
38
40
Krypton is primarily used in which of the following applications?
Fuel
Lighting
Solvent
Fertilizer
Which isotopic form of krypton is used in the dating of ancient ice cores?
Krypton-81
Krypton-84
Krypton-86
Krypton-82
Which of the following properties of krypton is true?
It has a high reactivity with metals.
It has a melting point of -157.36 °C.
It is a good conductor of electricity.
It is commonly found in the Earth's crust.
What is the state of krypton at room temperature?
Solid
Liquid
Gas
Plasma
Krypton is found in the Earth's atmosphere at a concentration of approximately:
0.0001%
1%
10%
20%
The discovery of krypton was made in which year?
1898
1905
1920
1930
Krypton forms compounds with which of the following elements?
Fluorine
Carbon
Oxygen
Nitrogen
In medical applications, krypton is used in:
MRI scanning
X-ray machines
Lasers for eye surgery
Antiseptics
Krypton's name is derived from the Greek word "kryptos," which means:
Invisible
Powerful
Heavy
Light
Before you leave, take our quick quiz to enhance your learning!

