What is the primary use of lead in modern industries?
Batteries
Pharmaceuticals
Food preservation
Cosmetics
Lead, a heavy metal with significant historical and contemporary importance, is a topic of great relevance in various educational fields. Its properties, uses, and health implications offer a rich subject for teachers to explore in the classroom. This guide provides educators with practical examples and applications of Lead, making it easier to convey its complex characteristics to students. From discussing its role in batteries to examining its environmental impact, this guide is designed to enrich educational discussions about Lead, fostering a deeper understanding among learners.
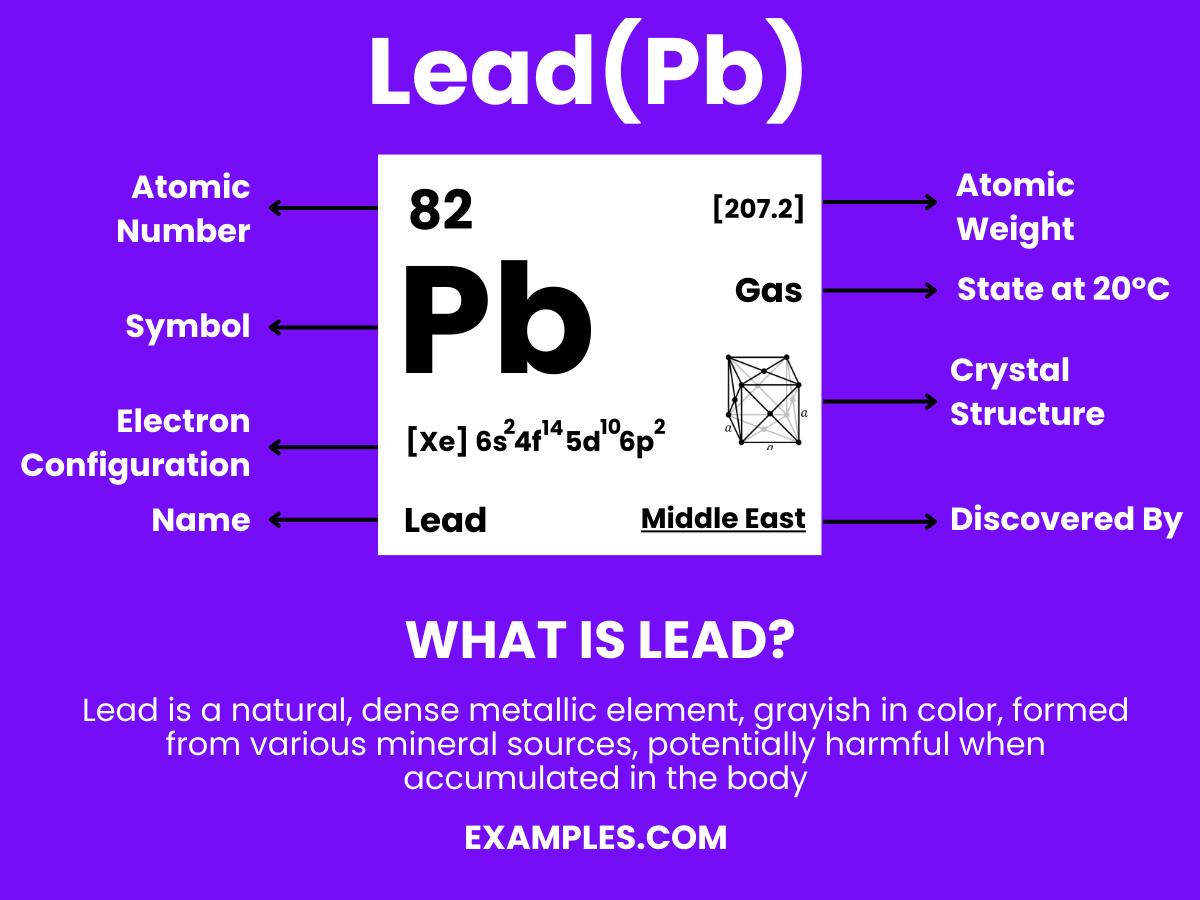
Lead is a dense, soft, and malleable metal with the symbol Pb (from the Latin plumbum) and atomic number 82. Historically used in a range of applications, Lead is known for its low melting point, high density, and resistance to corrosion. However, its use has decreased due to health concerns, as Lead exposure can be harmful. In the educational context, Lead serves as an important example of the intersection between chemistry, history, and environmental science, offering a multifaceted topic for teachers to delve into with their students.
Lead, symbolized as Pb, is a chemical element with fascinating properties and extensive uses. Its atomic structure offers insight into its unique characteristics. Here’s a point-wise breakdown of lead’s atomic structure:
1. Proton Count: Each lead atom contains 82 protons in its nucleus. This proton count is responsible for its chemical properties and its position as element 82 on the periodic table.
2. Neutron Variability: Lead has varying numbers of neutrons across its isotopes, which affects its atomic mass. The most common isotopes of lead are Pb-204, Pb-206, Pb-207, and Pb-208, with neutron counts that contribute to the overall stability of each isotope.
3. Electron Configuration: Lead’s electron configuration is [Xe] 4f14 5d10 6s2 6p2, indicating it has electrons in several energy levels. This configuration is crucial for understanding lead’s chemical behavior and bonding characteristics.
4. Metallic Bonding: In solid lead, atoms are bound together in a metallic lattice structure. This structure involves a ‘sea’ of delocalized electrons that are not associated with any one atom but move freely throughout the metal. This feature is key to lead’s conductivity and malleability.
5. Atomic Mass: The atomic mass of lead varies with its isotopes, with a standard atomic weight of about 207.2 u. This variation is due to the different numbers of neutrons in each isotope.
6. Chemical and Physical Properties: The atomic structure contributes to lead’s dense, soft nature, and its low melting point. These properties, combined with its resistance to corrosion, make lead suitable for a wide range of applications.
7. Applications of Lead :Due to its atomic structure and resultant physical properties, lead is extensively used in batteries, radiation shielding, and as an additive in alloys. Its ability to absorb vibration also makes it useful in soundproofing applications.
| Property | Description |
|---|---|
| Appearance | Silvery, bluish-gray metal |
| Atomic Mass | Average atomic mass of approximately 207.2 u |
| Density | Approximately 11.34 g/cm³ at room temperature |
| Melting Point | 327.46°C (621.43°F) |
| Boiling Point | 1749°C (3180°F) |
| Electrical Conductivity | Poor conductor of electricity compared to other metals |
| Thermal Conductivity | Relatively low; about 35 W/(m·K) |
| Malleability and Ductility | Highly malleable and ductile; can be shaped into various forms |
| Crystal Structure | Face-centered cubic (fcc) |
Lead exhibits a range of chemical properties that are crucial for its applications and interactions with other substances. Below are some of the key chemical properties of lead:
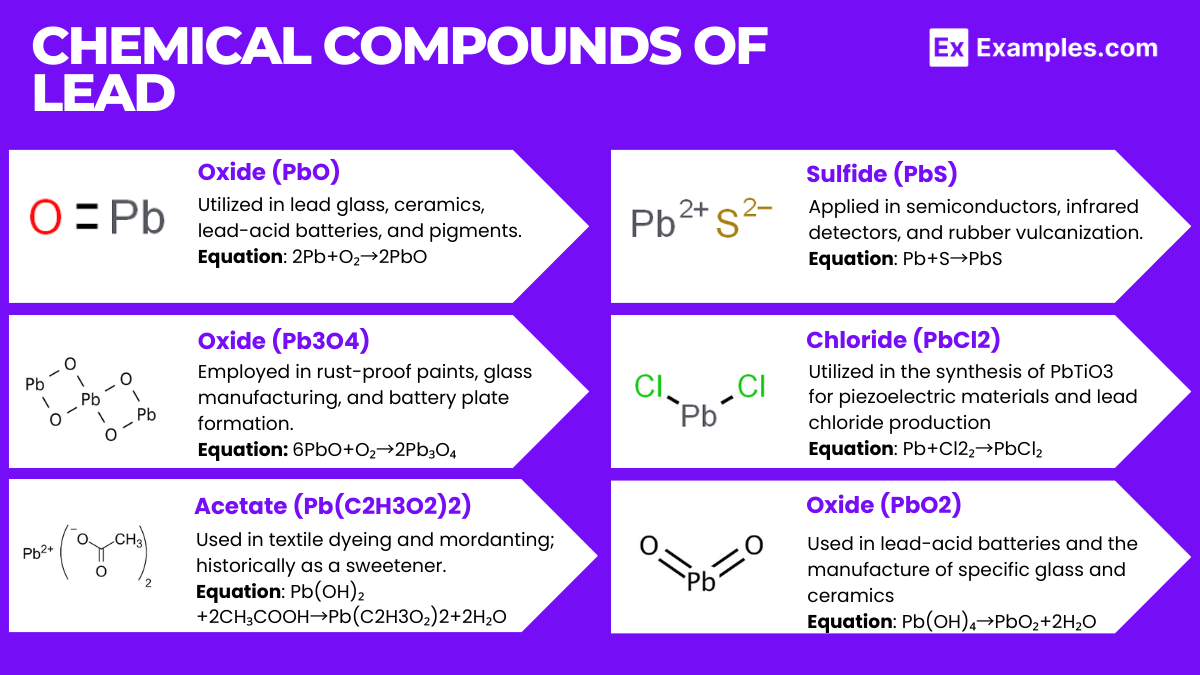
Lead reacts with oxygen to form lead oxide. Upon exposure to air, lead forms a protective layer of oxide that inhibits further corrosion. The reaction can be represented as: 2Pb + O₂→2PbO
Reaction with Acids: Lead is relatively resistant to corrosion in sulfuric and hydrochloric acid due to the formation of a protective layer.
However, it dissolves in nitric acid, forming lead nitrate: Pb + 4HNO₃→Pb(NO₃)₂+2NO₂+2H₂O
Reaction with Sulfur: Lead reacts with sulfur to form lead sulfide, a reaction that is of particular importance in the processing of lead ores: Pb + S→PbS
Solubility: Lead compounds exhibit varied solubility in water. Lead(II) chloride (PbCl2) is sparingly soluble, while lead(II) acetate (Pb(C₂H₃O₂)₂) is soluble, making it toxic when it leaches into water supplies.
Oxidation States: Lead primarily exhibits two oxidation states: +2 (lead(II)) and +4 (lead(IV)). Lead(II) compounds are more stable and common, such as PbO, PbS, and PbCO₃. Lead(IV) compounds are strong oxidizing agents and less stable, such as PbO₂.
Environmental Impact: Lead compounds can have significant environmental and health impacts. For example, lead(II) acetate is toxic, and lead oxides can contribute to air and water pollution, affecting human health and the ecosystem.
Lead has four stable isotopes, which are crucial for various scientific and practical applications. These isotopes differ in the number of neutrons in their nuclei, giving each a unique atomic mass. The stable isotopes of lead include:
Lead is utilized in various applications, benefiting from its physical and chemical properties. Some of the primary uses include:
The commercial production of lead primarily involves the processing of lead ore, with the two most common ores being galena (lead sulfide, PbS) and, to a lesser extent, cerussite (lead carbonate, PbCO3). The production process consists of several steps designed to extract and purify lead:
This production process yields lead that is ready for commercial use in batteries, radiation shielding, and other applications
Lead exposure can have severe health effects, particularly on young children and pregnant women. Even at low levels, lead exposure can cause a range of health issues:
Lead, symbolized as Pb, is a versatile metal with significant industrial applications, from batteries to radiation shielding. Despite its utility, lead poses serious health risks, including neurological damage and cardiovascular issues. Responsible use, recycling, and minimizing exposure are crucial to harnessing lead’s benefits while protecting public health and the environment.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary use of lead in modern industries?
Batteries
Pharmaceuticals
Food preservation
Cosmetics
What health problem is majorly associated with lead exposure?
Dermatitis
Asthma
Lead poisoning
Diabetes
Which property makes lead suitable for use in radiation shielding?
Transparency
High density
Solubility
Elasticity
In which form is lead often recycled?
Sheets
Pipes
Bullets
Batteries
What is a historical use of lead that is no longer common due to health concerns?
Jewelry making
Paint additive
Beverage sweetener
Fuel additive
Which agency regulates lead exposure levels in the workplace in the United States?
FDA
EPA
OSHA
USDA
What environmental issue is exacerbated by improper disposal of lead-based products?
Air pollution
Water pollution
Soil contamination
Noise pollution
What is a common symptom of acute lead poisoning in children?
Coughing
Rash
Irritability
Sneezing
How is lead most commonly ingested in contaminated environments?
Inhalation of dust
Consumption of contaminated food
Skin contact
Drinking contaminated water
What method is often used to remove lead from drinking water systems?
Chlorination
Filtration
Boiling
UV treatment
Before you leave, take our quick quiz to enhance your learning!

