What is the atomic number of lithium?
1
2
3
4
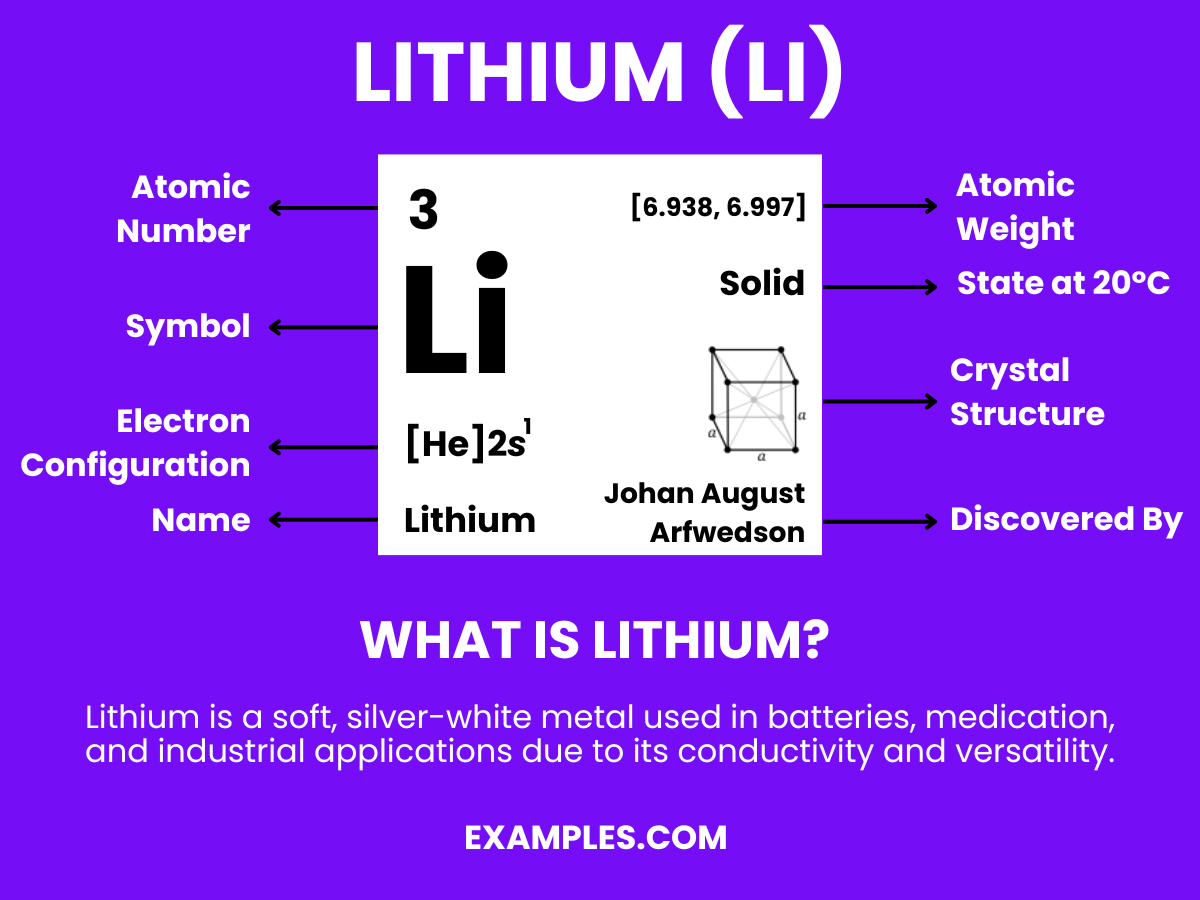
Lithium, the lightest metal and a cornerstone in modern chemistry, holds significant importance in various applications from batteries to mental health. This guide provides an in-depth look into the world of lithium, exploring its fundamental properties, uses, and safety measures. Especially tailored for educators, it offers practical examples and tips to effectively teach and communicate the versatile roles of lithium in both everyday life and advanced scientific research.
Lithium is a soft, silvery-white alkali metal, known for being the lightest metal and solid element under standard conditions. It’s highly reactive and flammable, often stored in mineral oil. Lithium is crucial in various applications, including rechargeable batteries, mental health medication, and as a heat transfer agent. Its unique properties make it a subject of interest in both educational and industrial settings.
| Sodium |
| Potassium |
| Rubidium |
| Cesium |
| Francium |
Formula: Li
Composition: A single lithium atom.
Bond Type: Highly reactive, especially with water.
Molecular Structure: Soft metal.
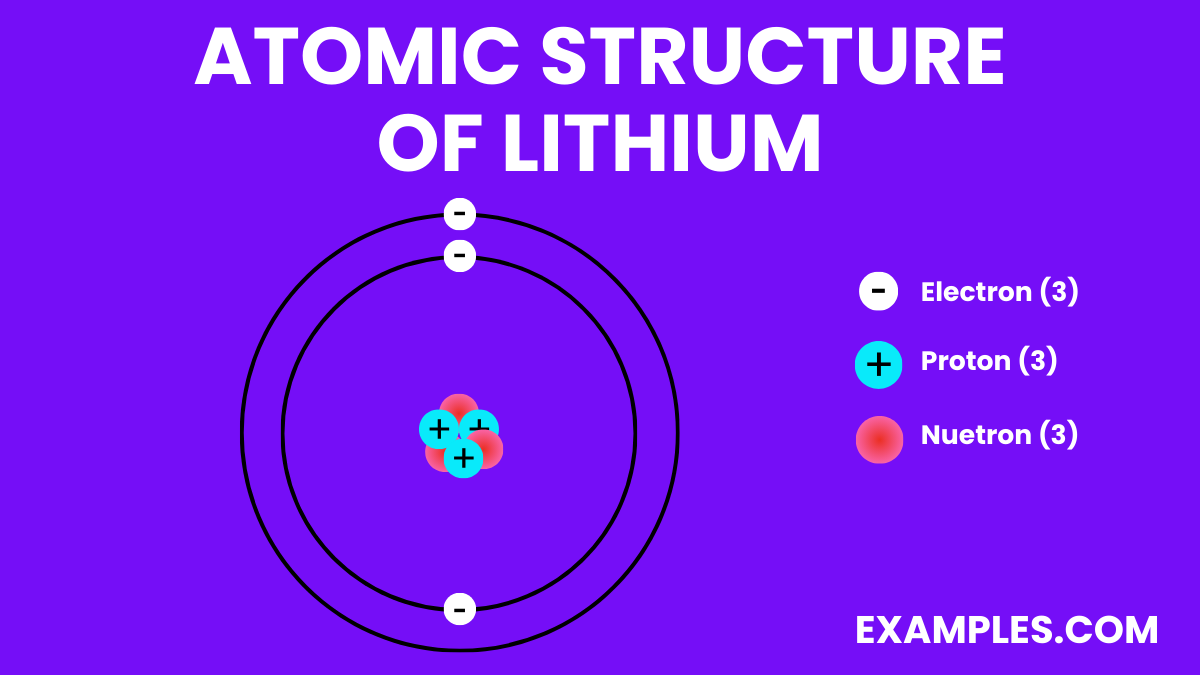
Electron Configuration: 3 electrons; configuration 1s² 2s¹.
Significance: Used in rechargeable batteries and mental health treatment.
Role in Chemistry: Reacts vigorously, forming compounds like lithium oxide (Li₂O).
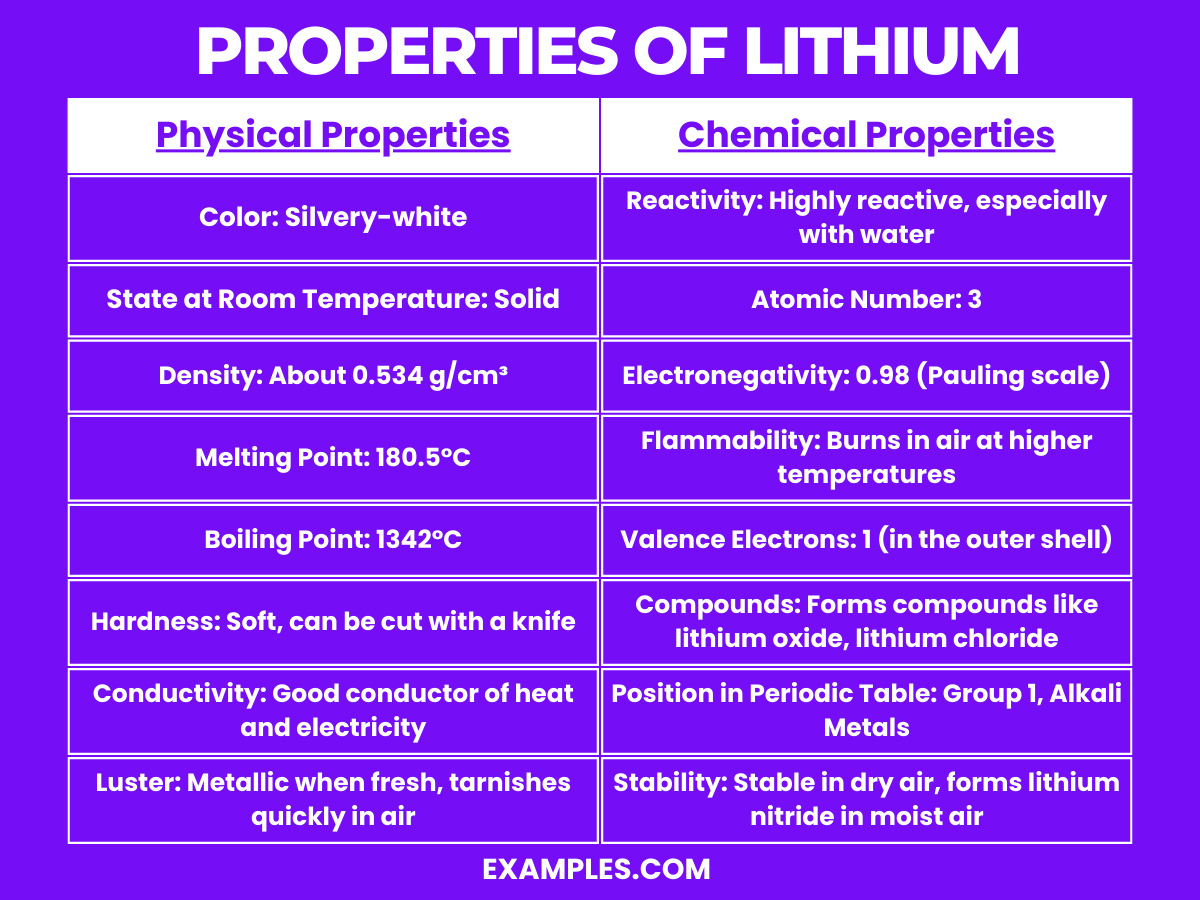
| Physical Property | Description |
|---|---|
| Color | Silvery-white |
| State at Room Temperature | Solid |
| Density | About 0.534 g/cm³ |
| Melting Point | 180.5°C (356.9°F) |
| Boiling Point | 1342°C (2448°F) |
| Hardness | Soft, can be cut with a knife |
| Conductivity | Good conductor of heat and electricity |
| Luster | Metallic when fresh; tarnishes quickly in air |
Lithium, the lightest metal, exhibits several unique chemical properties:
These chemical properties make lithium a highly interesting and important element in both industrial applications and chemical education.
| Property | Value |
|---|---|
| Atomic Number | 3 |
| Atomic Weight | 6.94 g/mol |
| Phase at Room Temperature | Solid |
| Density | 0.534 g/cm³ |
| Melting Point | 180.54°C (356.97°F) |
| Boiling Point | 1342°C (2448°F) |
| Specific Heat Capacity (Cp) | 3.58 J/(g·K) at 25°C |
| Thermal Conductivity | 84.8 W/(m·K) |
| Heat of Fusion | 3.00 kJ/mol |
| Heat of Vaporization | 145.92 kJ/mol |
| Property | Value |
|---|---|
| Crystal Structure | Body-Centered Cubic (bcc) |
| Electrical Resistivity | 92.8 nΩ·m at 20°C |
| Young’s Modulus | 4.9 GPa |
| Shear Modulus | 4.2 GPa |
| Bulk Modulus | 11 GPa |
| Poisson’s Ratio | 0.36 |
| Mohs Hardness | 0.6 |
| Brinell Hardness | 5 MPa |
| Thermal Expansion Coefficient | 46 µm/(m·K) at 25°C |
| Electronegativity (Pauling scale) | 0.98 |
| Property | Value |
|---|---|
| Electrical Resistivity | 92.8 nΩ·m at 20°C |
| Electronegativity (Pauling scale) | 0.98 |
| Thermal Conductivity | 84.8 W/(m·K) |
| Ionization Energy | First: 520.2 kJ/mol |
| Property | Value |
|---|---|
| Isotopes | Lithium-6 (⁶Li), Lithium-7 (⁷Li) |
| Natural Abundance | ⁶Li: 7.5%, ⁷Li: 92.5% |
| Nuclear Spin | ⁶Li: 1, ⁷Li: 3/2 |
| Neutron Cross Section | ⁶Li: 940 barns, ⁷Li: 0.045 barn |
| Stable Isotopes | ⁶Li, ⁷Li |
| Application | ⁶Li is used in nuclear fusion and as a neutron absorber in nuclear reactors. ^7Li is used in the production of lithium batteries. |
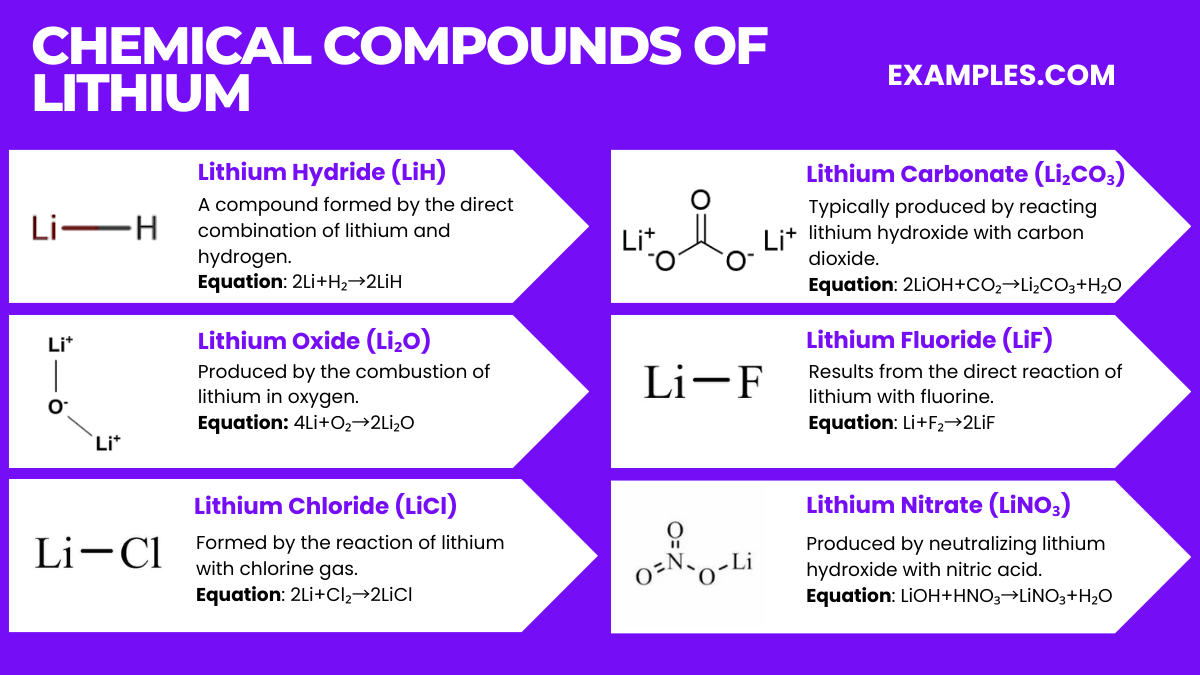
Lithium forms various compounds, widely used in industry and technology. Here are six key lithium compounds along with their chemical equations:
Lithium has several isotopes, each with unique properties. The table below provides an overview:
| Isotope | Mass Number | Natural Abundance (%) | Half-Life | Decay Mode |
|---|---|---|---|---|
| Li-6 | 6 | 7.5 | Stable | – |
| Li-7 | 7 | 92.5 | Stable | – |
| Li-8 | 8 | Trace | 0.84 seconds | Beta decay |
| Li-9 | 9 | Synthetic | 178.3 ms | Beta decay |
| Li-10 | 10 | Synthetic | 2 ms | Beta- and neutron emission |
| Li-11 | 11 | Synthetic | 8.75 ms | Beta decay |
The most common isotopes are Lithium-6 and Lithium-7, with Lithium-7 being the predominant isotope found in nature. The heavier isotopes, like Lithium-8 and beyond, are unstable and decay rapidly. These isotopes are primarily of interest in nuclear physics and related research fields.

Lithium, a versatile element, is employed in various applications:
The commercial production of lithium is mainly from mineral deposits and brine water:
Lithium, primarily used in medical applications and various industries, has several health effects:
The environmental impact of lithium is primarily associated with its extraction and usage:
Lithium is widely used in rechargeable batteries, psychiatric medication, manufacturing strong but lightweight alloys, and in glass and ceramics production.
In high doses, lithium can be toxic, causing side effects like nausea, tremors, and kidney issues, necessitating careful medical monitoring.
Lithium is typically found in underground mineral deposits and in saline lake brines, predominantly in South America and Australia.
Lithium is the 3rd element in the periodic table due to its atomic number, which signifies it has three protons in its nucleus.
Lithium is a soft, silvery-white alkali metal, known for its high reactivity and being the lightest metal in the periodic table.
Lithium, a key element in modern technology and medicine, holds significant importance due to its unique properties. Its role in powering batteries, treating bipolar disorder, and enhancing material strength underscores its versatility. Understanding lithium’s applications, health implications, and environmental impact is essential, highlighting the need for responsible usage and sustainable practices in its extraction and application.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of lithium?
1
2
3
4
Lithium is part of which group on the periodic table?
Alkali metals
Alkaline earth metals
Transition metals
Halogens
Which of the following is a common use of lithium?
In fireworks
In batteries
In glass making
All of the above
Lithium reacts with water to form which of the following?
Lithium hydroxide
Lithium chloride
Lithium carbonate
Lithium nitrate
Which isotope of lithium is more abundant in nature?
Lithium-6
Lithium-7
Lithium-8
Lithium-9
What property of lithium makes it useful in treating bipolar disorder?
Its reactivity
Its density
Its mood-stabilizing properties
Its conductivity
How does lithium react with nitrogen?
Forms lithium nitride
Forms lithium nitrate
Does not react
Forms lithium oxide
Lithium metal is stored under which of the following conditions?
Exposed to air
Submerged in oil
Refrigerated
Heated
Lithium is primarily extracted from which source?
Petroleum
Coal
Mineral springs
Seawater
What is the effect of lithium on flames when used in fireworks?
Red color
Green color
Blue color
White color
Before you leave, take our quick quiz to enhance your learning!

