What is the chemical formula of magnesium sulfate?
MgSO4
MgSO3
MgS2O4
Mg(SO4)2

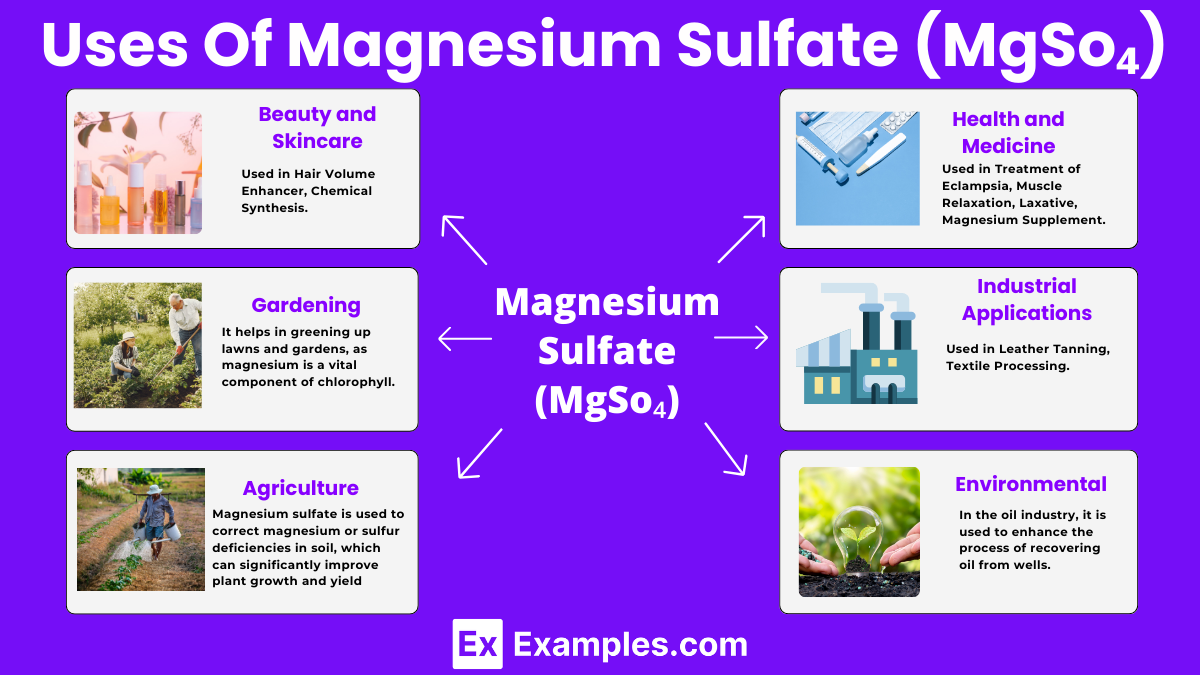
Magnesium sulfate, known by the chemical formula MgSO₄, is a chemical compound widely recognized for its diverse applications in agriculture, medicine, and industry. This compound typically appears as a white crystalline solid and is highly soluble in water, forming a clear solution. Magnesium sulfate plays a pivotal role in healthcare as a treatment for various conditions, including magnesium deficiency, eclampsia during pregnancy, and as a laxative. It is also used in agriculture to enrich soils deficient in magnesium, an essential nutrient for plant growth. Additionally, magnesium sulfate is utilized in several industrial processes, including the preparation of textiles and in the manufacture of certain cements, showcasing its versatility across different fields.
Magnesium sulfate is a compound consisting of magnesium, sulfur, and oxygen, with the formula MgSO₄. It often occurs with varying amounts of water, forming hydrates such as the commonly known Epsom salt (MgSO₄·7H₂O). At the molecular level, magnesium sulfate is made up of magnesium ions (Mg²⁺) and sulfate ions (SO₄²⁻). This ionic structure enables it to dissolve readily in water, as the polar water molecules interact with and separate the magnesium and sulfate ions.
In its most familiar form as Epsom salt, magnesium sulfate forms an orthorhombic crystal structure, providing its characteristic appearance. This crystalline form is important for its use in bath salts, therapeutic soaks, and in agriculture as a magnesium supplement for plants.
| Property | Value |
|---|---|
| Formula | MgSO₄ |
| Hill Formula | MgO₄S |
| Name | Magnesium sulfate |
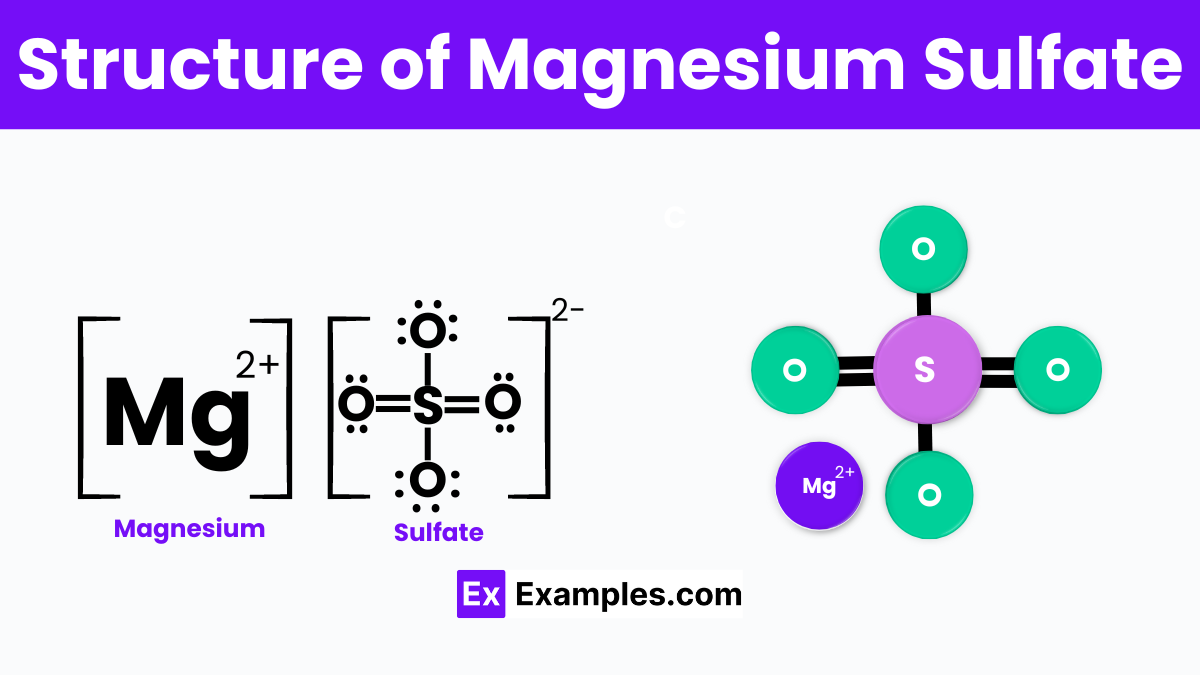
The structure of magnesium sulfate (MgSO₄) is characterized by the central magnesium ion (Mg²⁺) which is bonded to four oxygen atoms (O) from the sulfate ion (SO₄²⁻), forming a tetrahedral geometry. This arrangement allows magnesium sulfate to readily dissolve in water, as the polar water molecules interact with both the magnesium and sulfate ions. In its most common form, magnesium sulfate appears as a white or colorless crystalline solid. When hydrated, which is often the case with Epsom salt (MgSO₄·7H₂O), it incorporates seven water molecules into its crystalline structure, further influencing its solubility and reactivity. The ability of magnesium sulfate to form different hydrates contributes to its versatility in various applications, from medical treatments to agricultural uses.
Magnesium sulfate can be prepared through several methods, with one of the most common being the reaction of magnesium oxide (MgO) or magnesium carbonate (MgCO₃) with sulfuric acid (H₂SO₄). The general equation for producing magnesium sulfate from magnesium oxide is as follows:
Similarly, when magnesium carbonate reacts with sulfuric acid, magnesium sulfate, carbon dioxide, and water are produced:
These reactions are exothermic and result in the formation of magnesium sulfate, which can then be crystallized as the heptahydrate form (Epsom salt) by cooling and allowing the water to evaporate, leading to the precipitation of the hydrated salt. This method is practical for both laboratory-scale and industrial production of magnesium sulfate.
| Property | Description |
|---|---|
| Molecular Formula | MgSO4 |
| Appearance | White or colorless crystalline solid |
| Molar Mass | 120.366 g/mol (anhydrous), 246.47 g/mol (heptahydrate) |
| Density | 2.66 g/cm³ (anhydrous), 1.68 g/cm³ (heptahydrate) |
| Melting Point | 1,124°C (anhydrous), 150°C (heptahydrate, decomposes) |
| Solubility in Water | 35.1 g/100 mL (20°C, anhydrous), Highly soluble (heptahydrate) |
| Hygroscopicity | Yes, especially the heptahydrate form |
| Crystal Structure | Orthorhombic (anhydrous), Monoclinic (heptahydrate) |
Magnesium sulfate consists of one magnesium ion (Mg²⁺) and one sulfate ion (SO₄²⁻). It commonly occurs as its heptahydrate form, MgSO₄·7H₂O, known as Epsom salt.
Magnesium sulfate is hygroscopic, absorbing moisture from the air, which makes it an excellent drying agent in various chemical processes.
Magnesium sulfate heptahydrate loses water upon heating, illustrating a stepwise dehydration process to form anhydrous MgSO₄.
This reaction is pivotal in drying and dehydration processes in the chemical industry.
An aqueous solution of magnesium sulfate is nearly neutral, which is significant for applications that require a neutral pH environment.
| Property | Value |
|---|---|
| CAS Registry Number | 7487-88-9 |
| PubChem Compound ID | 24083 |
| PubChem Substance ID | 24852194 |
| SMILES Identifier | [O-]S(=O)(=O)[O-].[Mg+2] |
| InChI Identifier | InChI=1/Mg.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)/q+2;/p-2/fMg.O4S/qm;-2 |
| InChI Key | WRUGWIBCXHJTDG-QMFXNGBRCP |
| RTECS Number | OM4500000 |
| MDL Number | MFCD00011110 |
Although rare, some people may experience allergic reactions to magnesium sulfate, which can include:
Yes, magnesium sulfate is commonly known as Epsom salt, widely used for baths to relieve muscle aches and stress.
Individuals with kidney disease, severe dehydration, or certain heart conditions should avoid taking magnesium sulfate.
Magnesium sulfate is given in pregnancy to prevent seizures in women with pre-eclampsia or to treat eclampsia, ensuring maternal and fetal safety.
A pregnant woman can be on magnesium sulfate for up to 48 hours to manage pre-eclampsia or prevent eclampsia-related seizures.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical formula of magnesium sulfate?
MgSO4
MgSO3
MgS2O4
Mg(SO4)2
Magnesium sulfate is commonly used in which medical treatment?
Pain relief
Muscle relaxation
Antibiotic therapy
Antifungal treatment
In which form is magnesium sulfate commonly administered for medical use?
Oral tablets
Injection
Cream
Nasal spray
What is a common agricultural use of magnesium sulfate?
Fertilizer
Pest control
Soil acidity adjustment
Seed treatment
How does magnesium sulfate function as a laxative?
It absorbs water in the intestines
It increases peristalsis in the intestines
It reduces intestinal absorption
It creates a barrier in the intestines
What is the primary role of magnesium in the body?
Blood clotting
Bone strength
Oxygen transport
Digestion
What is the appearance of magnesium sulfate in its hydrated form?
White crystalline powder
Yellow liquid
Blue crystals
Green powder
Magnesium sulfate is used in the treatment of which pregnancy-related condition?
Morning sickness
Preterm labor
Gestational diabetes
Eclampsia
How is magnesium sulfate used in the treatment of asthma?
As a bronchodilator
To decrease airway inflammation
To relax the bronchial muscles
To increase mucus production
What precaution should be taken when administering magnesium sulfate intravenously?
Monitor blood glucose levels
Ensure adequate hydration
Monitor blood pressure and heart rate
Administer with high doses of vitamin
Before you leave, take our quick quiz to enhance your learning!

