What is the molecular formula of methane?
CH₃
CH₄
C₂H₄
C₂H₆

Methane is a simple but very important covalent compound in the world of chemistry. It consists of one carbon atom bonded tightly to four hydrogen atoms. This gas can be found in natural environments, such as swamps and landfills, and is also a major component of natural gas, making it a key source of energy for heating and cooking in our homes. The study of methane helps us understand the basics of chemical bonds and how elements come together to form compounds. By exploring methane, we gain insights into both the natural processes that produce it and the human activities that release it into the atmosphere, highlighting its significance in our daily lives and the environment.
Methane(CH₄) is a colorless, odorless gas that plays a crucial role in both the environment and the energy sector. It is the simplest form of a hydrocarbon, consisting of one carbon atom bonded to four hydrogen atoms (CH₄). This covalent compound is a primary component of natural gas, making it a significant source of fuel used for heating, cooking, and generating electricity. Methane is also naturally produced through the decomposition of organic matter in environments like wetlands and as a byproduct of digestive processes in certain animals. Due to its potent greenhouse effect, methane is also a key focus in discussions about climate change and environmental protection.
| Property | Value |
|---|---|
| Formula | CH₄ |
| Name | Methane |
| Alternate Names | Biogas, Fire Damp, Freon 50, Marsh Gas, Methyl Hydride, R-50, Carbane |
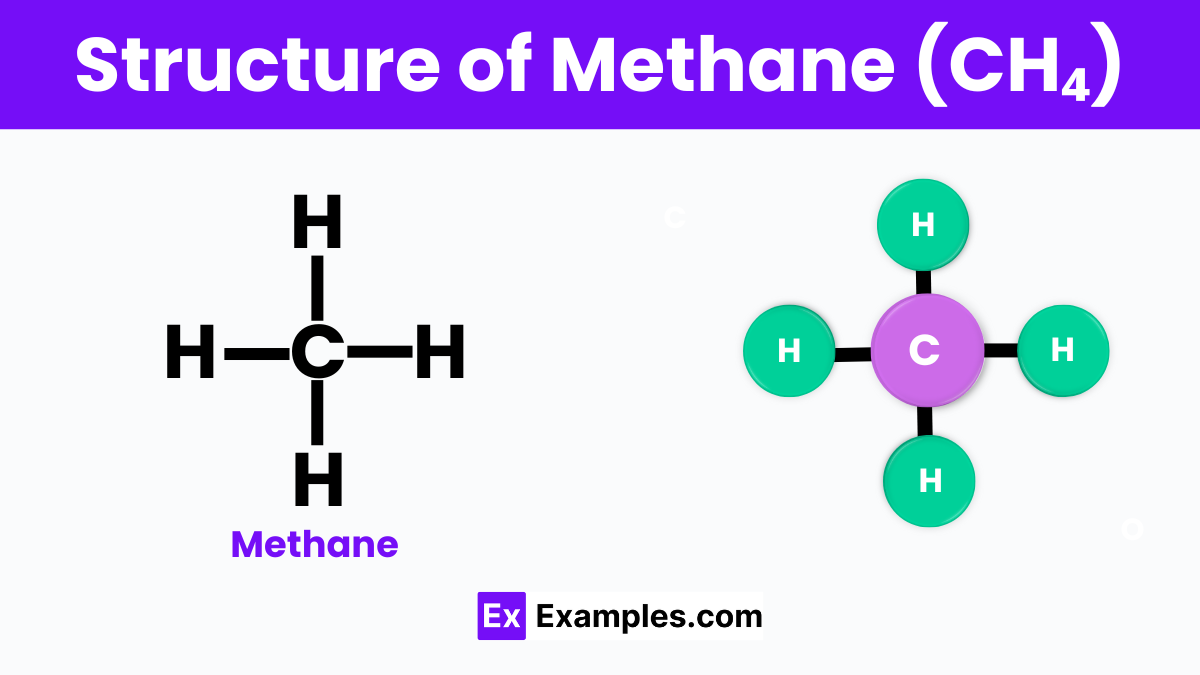
The structure of methane is characterized by its simple and symmetric shape, where one carbon atom sits at the center, bonded to four hydrogen atoms in a tetrahedral arrangement. This configuration gives methane its stability and gaseous state at room temperature. The bonds between the carbon and hydrogen atoms are covalent, meaning they share electrons to form a strong connection. This tetrahedral structure is a fundamental concept in chemistry, illustrating how atoms can come together to form molecules in a specific geometric pattern.
The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable.
Decarboxylation of Sodium Acetate: This process involves heating sodium acetate with soda lime (a mixture of NaOH and CaO). Soda lime acts as a catalyst in the reaction and facilitates the removal of a carbon dioxide molecule from sodium acetate, resulting in the formation of methane. The chemical equation for this reaction is as follows:
Hydrolysis of Aluminium Carbide: Another method to prepare methane is by reacting aluminium carbide (3Al₄C₃) with water. This reaction produces methane gas and aluminium hydroxide as by-products. The reaction can be represented by the following chemical equation
These methods demonstrate the versatile approaches to synthesizing methane, highlighting its significance in both industrial applications and laboratory settings.
| Property | Value/Description |
|---|---|
| Molecular Formula | CH₄ |
| Molecular Weight | 16.04 g/mol |
| State at Room Temperature | Gas |
| Color | Colorless |
| Odor | Odorless |
| Boiling Point | -161.5 °C (111.65 K) |
| Melting Point | -182.5 °C (90.65 K) |
| Density | 0.656 kg/m³ (gas at 0°C and 1 atm) |
| Solubility in Water | Low |
| Specific Gravity | 0.554 (air=1) |
| Heat of Combustion | -890 kJ/mol |
Methane combusts in the presence of oxygen to produce carbon dioxide and water, releasing a significant amount of energy. This reaction is fundamental to methane’s use as a fuel.
Equation: CH₄ + 2O₂ → CO₂ + 2H₂O + Energy
Methane reacts with halogens under specific conditions in a substitution reaction, where hydrogen atoms are replaced by halogen atoms. For example, the reaction with chlorine in the presence of light or heat produces chloromethane and hydrochloric acid.
Equation: CH₄ + Cl₂ → CH₃Cl + HCl
When methane is subjected to high temperatures (usually in the absence of oxygen), it decomposes into hydrogen and solid carbon. This process is known as pyrolysis or cracking.
Equation: CH₄ → C + 2H₂
Methane reacts with steam at high temperatures to produce carbon monoxide and hydrogen. This reaction is critical in the industrial production of hydrogen and synthesis gas (syngas).
Equation: CH₄ + H₂O → CO + 3H₂
Methane can react with oxygen-containing compounds under certain conditions. For example, with oxygen, it can form methanol, although this requires a catalyst and high pressure.
Equation: CH₄ + O₂ → CH₃OH
| Property | Value |
|---|---|
| CAS Registry Number | 74-82-8 |
| Beilstein Number | 1718732 |
| PubChem Compound ID | 297 |
| PubChem Substance ID | 24857787 |
| SMILES Identifier | C |
| InChI Identifier | InChI=1/CH4/h1H4 |
| RTECS Number | PA1490000 |
| MDL Number | MFCD00008279 |
| Property | Value |
|---|---|
| NFPA Health Rating | 2 |
| NFPA Fire Rating | 4 |
| NFPA Reactivity Rating | 0 |

Methane is a primary component of natural gas, making it a crucial energy source for heating, cooking, and electricity generation due to its high combustion efficiency.
It serves as a key raw material in the production of hydrogen, methanol, and other chemicals through processes like steam reforming and methanol synthesis.
Compressed natural gas (CNG), which is primarily methane, is used as a cleaner alternative to gasoline and diesel in vehicles.
Methane is a starting material in the Haber process for synthesizing ammonia, which is then used to produce fertilizers.
Methane is generated as a byproduct of organic waste decomposition in landfills and can be captured to use as a renewable energy source, reducing greenhouse gas emissions.
Methane captured from biogas facilities (from waste treatment plants or agricultural waste) can be used to generate electricity in power plants.
Methane is a key component of natural gas, used widely for generating electricity and heating, offering a cleaner burn compared to other fossil fuels.
Converted into CNG or LNG, methane serves as a cleaner alternative to gasoline and diesel for vehicles, reducing air pollution.
As a vital raw material in the chemical industry, methane is used to produce essential chemicals for plastics, synthetic fibers, and pharmaceuticals.
Capturing methane from landfills allows its use as an energy source, reducing greenhouse gas emissions and harnessing value from waste.
Through biogas production from organic waste, methane provides renewable energy for electricity, heating, or fuel, contributing to sustainable agriculture.
Methane is widely used for cooking and heating due to its efficiency and clean combustion, making it a preferred choice in households.
The extraction and processing of methane create jobs and foster economic growth, particularly in methane-producing regions.
Methane enhances energy security by diversifying energy sources and reducing reliance on oil, contributing to national stability.
Methane is a potent greenhouse gas, contributing to climate change more significantly than CO₂ over the short term, mainly due to emissions from agriculture, waste management, and fossil fuels.
Leaking methane can form ground-level ozone, reducing air quality, affecting human health, agricultural productivity, and ecosystems.
High methane levels can displace oxygen, posing suffocation risks, especially in confined areas.
Leaks from gas systems and landfills can harm local ecosystems and waste energy that could be used for heating or electricity.
Methane’s flammability presents risks of explosions and fires in areas where it accumulates, necessitating stringent management to prevent accidents.
Methane in high concentrations can displace oxygen, potentially causing suffocation. Normally, it’s non-toxic and poses little direct risk at low levels.
Methane is found in natural gas deposits, landfills, wetlands, and as a byproduct of agricultural activities, especially from livestock.
Methane is a potent greenhouse gas, contributing significantly to global warming by trapping heat in the atmosphere more effectively than CO2.
Methane is odorless. The distinctive smell associated with natural gas is added for safety reasons to detect leaks.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the molecular formula of methane?
CH₃
CH₄
C₂H₄
C₂H₆
Methane is classified as which type of hydrocarbon?
Alkene
Alkyne
Alkane
Aromatic
Methane is a major component of which natural resource?
Coal
Petroleum
Natural gas
Biomass
What is the bond angle in a methane molecule?
90°
109.5°
120°
180°
What type of bond exists between the carbon and hydrogen atoms in methane?
Ionic
Metallic
Covalent
Hydrogen
Methane is a greenhouse gas. How does its global warming potential compare to carbon dioxide over 100 years?
Lower
Similar
Higher
Negligible
Which process primarily produces methane in nature?
Photosynthesis
Respiration
Combustion
Anaerobic decomposition
What is the boiling point of methane at standard atmospheric pressure?
-161.5°C
-42°C
0°C
25°C
Methane can be used as a fuel. What is the primary product when methane combusts completely in oxygen?
Water only
Carbon monoxide and water
Carbon dioxide and water
Hydrogen and carbon dioxide
Which method is used to separate methane from natural gas?
Distillation
Crystallization
Filtration
Chromatography
Before you leave, take our quick quiz to enhance your learning!

