What is the primary function of myoglobin?
Transport oxygen in the blood
Store oxygen in muscle tissues
Carry carbon dioxide
Break down glucose


Myoglobin is a type of protein found in the muscle cells of many animals, including humans. Its main role is to store oxygen, which is crucial for muscles to function properly, especially during intense activity. Structurally, myoglobin is similar to a part of our blood called hemoglobin, which carries oxygen throughout our bodies. In chemistry, we refer to myoglobin as one of the complex compounds because it has a special component called heme, which binds oxygen molecules directly. Understanding how myoglobin works helps us appreciate how our bodies use oxygen to convert food into energy, keeping our muscles strong and healthy.
Deoxymyoglobin is the form of myoglobin when it is not bound to oxygen. This state is typically seen in muscle tissues that are at rest or not actively consuming oxygen. It has a purplish-red color.
Oxymyoglobin is formed when myoglobin binds to oxygen. It gives fresh muscle tissue its bright cherry-red color, commonly seen in the meat of recently slaughtered animals.
Metmyoglobin occurs when the iron in the myoglobin oxidizes further, losing its ability to bind oxygen. This form gives meat a brownish color and is often associated with meat that has been exposed to air for an extended period.

Myoglobin is a compact, globular protein that plays a crucial role in muscle tissue. At its core, it consists of a single polypeptide chain of 153 amino acids, which creates a structure with eight helical segments. Nestled within these helices is a heme group, a component crucial for binding oxygen. This heme group contains an iron atom at its center, which is where oxygen actually attaches. The overall structure of myoglobin allows it to efficiently store and release oxygen as needed by muscles, supporting sustained physical activity and energy production. This simple but effective design is essential for the oxygen supply to muscle cells, especially during intense exercise.
Myoglobin is classified as a globular protein, a type of protein that is soluble in water and has a spherical shape. This characteristic allows myoglobin to be highly functional within muscle cells, where it efficiently binds and releases oxygen. The globular nature of myoglobin makes it essential for its role in oxygen storage, providing the oxygen necessary for muscle cells to produce energy, especially during intense physical activities. This protein’s ability to bind oxygen is facilitated by its heme group, which contains an iron atom that directly interacts with oxygen molecules.
The molar mass of myoglobin is approximately 17,000 grams per mole. This measurement reflects the total mass of one mole of myoglobin molecules and serves as a way to express the mass of this protein for chemical calculations. The sum of the atomic masses of all the atoms in its amino acid chain and the critical heme group, which binds oxygen, determine the molar mass of myoglobin. This relatively moderate molar mass enables myoglobin to transport oxygen efficiently within muscle cells.
| Feature | Myoglobin | Hemoglobin |
|---|---|---|
| Function | Stores oxygen in muscle cells | Transports oxygen in the blood |
| Structure | Single polypeptide chain | Four polypeptide chains (two alpha and two beta) |
| Location | Found in muscle tissues | Found in red blood cells |
| Oxygen Binding | Binds oxygen tightly and releases it slowly | Binds oxygen less tightly, facilitating easy release |
| Color Change | Changes from purplish-red to bright red with oxygen binding | Changes from dark red to bright red with oxygen binding |
| Presence in Animals | Found in all vertebrates | Found in all vertebrates |
| Molecular Weight | Approximately 17,000 g/mol | Approximately 64,000 g/mol |
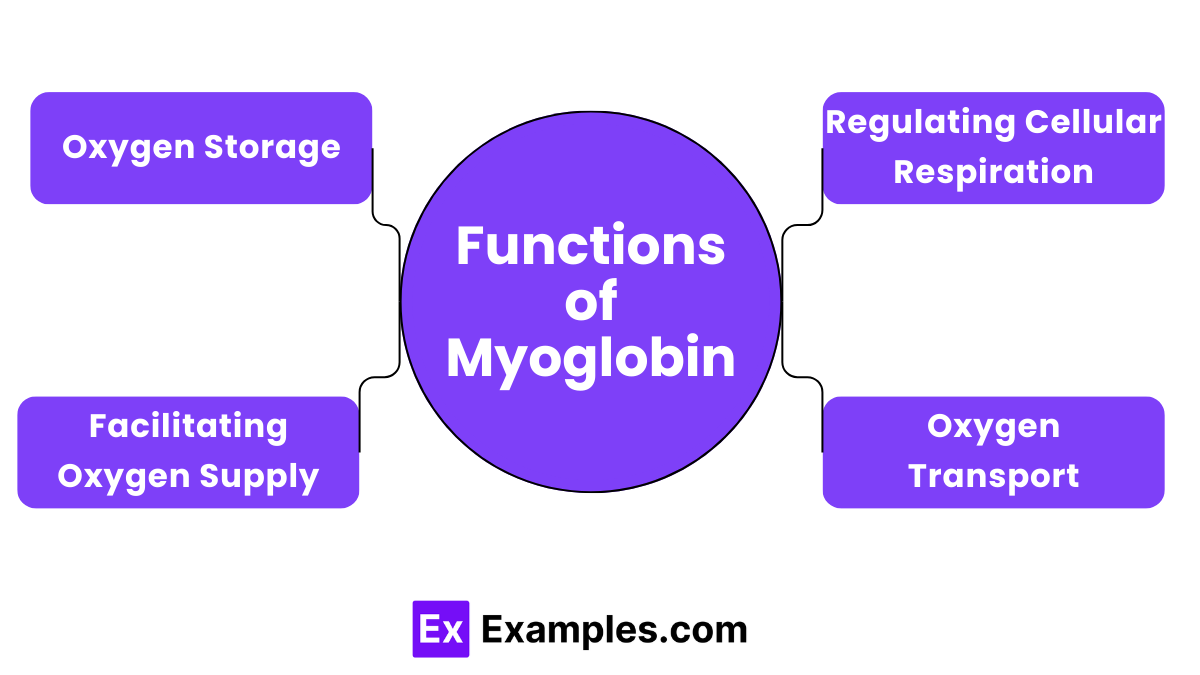
Myoglobin’s primary role is to store oxygen within muscle tissues. This function is crucial during times when cells require immediate oxygen, such as during intense physical activity.
Although its role is more limited compared to hemoglobin, myoglobin helps transport oxygen from the cell membrane to the mitochondria within muscle cells.
By storing oxygen, myoglobin ensures a steady supply to the mitochondria, which are crucial for sustained muscle activity and energy production.
Myoglobin plays a role in regulating the rate of cellular respiration by controlling the oxygen levels available for aerobic metabolism, supporting efficient energy use in muscle cells.
Myoglobin releases oxygen to muscle cells, aiding cellular respiration and energy production, enhancing physical performance.
Myoglobin is crucial for oxygen storage in muscles, ensuring continuous supply during high-demand activities like exercise.
Myoglobin is primarily located in muscle tissues, both cardiac and skeletal, supporting their high-energy demands.
Excessive myoglobin in the blood, usually from muscle damage, can lead to kidney injury by obstructing renal filtration systems.

Myoglobin is a type of protein found in the muscle cells of many animals, including humans. Its main role is to store oxygen, which is crucial for muscles to function properly, especially during intense activity. Structurally, myoglobin is similar to a part of our blood called hemoglobin, which carries oxygen throughout our bodies. In chemistry, we refer to myoglobin as one of the complex compounds because it has a special component called heme, which binds oxygen molecules directly. Understanding how myoglobin works helps us appreciate how our bodies use oxygen to convert food into energy, keeping our muscles strong and healthy.
Myoglobin is a protein found in muscle cells that helps store oxygen for when it’s needed most, like during intense exercise. It’s especially abundant in the muscles of mammals that dive deep underwater, such as whales and seals. The chemical formula of myoglobin is C₁₅₃₄H₂₄₄₁N₄₀₉O₄₄₂S₉Fe₁. This formula reflects its complex structure, which includes an iron-containing part called heme that binds oxygen. Myoglobin’s ability to bind oxygen tightly allows muscles to maintain high energy levels and endurance, supporting sustained physical activity.
Deoxymyoglobin is the form of myoglobin when it is not bound to oxygen. This state is typically seen in muscle tissues that are at rest or not actively consuming oxygen. It has a purplish-red color.
Oxymyoglobin is formed when myoglobin binds to oxygen. It gives fresh muscle tissue its bright cherry-red color, commonly seen in the meat of recently slaughtered animals.
Metmyoglobin occurs when the iron in the myoglobin oxidizes further, losing its ability to bind oxygen. This form gives meat a brownish color and is often associated with meat that has been exposed to air for an extended period.
Myoglobin is a compact, globular protein that plays a crucial role in muscle tissue. At its core, it consists of a single polypeptide chain of 153 amino acids, which creates a structure with eight helical segments. Nestled within these helices is a heme group, a component crucial for binding oxygen. This heme group contains an iron atom at its center, which is where oxygen actually attaches. The overall structure of myoglobin allows it to efficiently store and release oxygen as needed by muscles, supporting sustained physical activity and energy production. This simple but effective design is essential for the oxygen supply to muscle cells, especially during intense exercise.
Myoglobin is classified as a globular protein, a type of protein that is soluble in water and has a spherical shape. This characteristic allows myoglobin to be highly functional within muscle cells, where it efficiently binds and releases oxygen. The globular nature of myoglobin makes it essential for its role in oxygen storage, providing the oxygen necessary for muscle cells to produce energy, especially during intense physical activities. This protein’s ability to bind oxygen is facilitated by its heme group, which contains an iron atom that directly interacts with oxygen molecules.
The molar mass of myoglobin is approximately 17,000 grams per mole. This measurement reflects the total mass of one mole of myoglobin molecules and serves as a way to express the mass of this protein for chemical calculations. The sum of the atomic masses of all the atoms in its amino acid chain and the critical heme group, which binds oxygen, determine the molar mass of myoglobin. This relatively moderate molar mass enables myoglobin to transport oxygen efficiently within muscle cells.
Feature | Myoglobin | Hemoglobin |
|---|---|---|
Function | Stores oxygen in muscle cells | Transports oxygen in the blood |
Structure | Single polypeptide chain | Four polypeptide chains (two alpha and two beta) |
Location | Found in muscle tissues | Found in red blood cells |
Oxygen Binding | Binds oxygen tightly and releases it slowly | Binds oxygen less tightly, facilitating easy release |
Color Change | Changes from purplish-red to bright red with oxygen binding | Changes from dark red to bright red with oxygen binding |
Presence in Animals | Found in all vertebrates | Found in all vertebrates |
Molecular Weight | Approximately 17,000 g/mol | Approximately 64,000 g/mol |
Myoglobin’s primary role is to store oxygen within muscle tissues. This function is crucial during times when cells require immediate oxygen, such as during intense physical activity.
Although its role is more limited compared to hemoglobin, myoglobin helps transport oxygen from the cell membrane to the mitochondria within muscle cells.
By storing oxygen, myoglobin ensures a steady supply to the mitochondria, which are crucial for sustained muscle activity and energy production.
Myoglobin plays a role in regulating the rate of cellular respiration by controlling the oxygen levels available for aerobic metabolism, supporting efficient energy use in muscle cells.
Myoglobin releases oxygen to muscle cells, aiding cellular respiration and energy production, enhancing physical performance.
Myoglobin is crucial for oxygen storage in muscles, ensuring continuous supply during high-demand activities like exercise.
Myoglobin is primarily located in muscle tissues, both cardiac and skeletal, supporting their high-energy demands.
Excessive myoglobin in the blood, usually from muscle damage, can lead to kidney injury by obstructing renal filtration systems.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary function of myoglobin?
Transport oxygen in the blood
Store oxygen in muscle tissues
Carry carbon dioxide
Break down glucose
What metal ion is central to the myoglobin molecule?
Copper
Iron
Zinc
Magnesium
In which type of muscle is myoglobin most abundant?
Smooth muscle
Cardiac muscle
Skeletal muscle
Both cardiac and skeletal muscles
What color is myoglobin when it is oxygenated?
Blue
Red
Green
Yellow
Which of the following best describes the structure of myoglobin?
Multimeric
Dimeric
Monomeric
Trimeric
Myoglobin has a higher affinity for oxygen than hemoglobin at:
Low oxygen concentrations
High oxygen concentrations
Low carbon dioxide concentrations
High carbon dioxide concentrations
What is the molecular weight of myoglobin?
17 kDa
27 kDa
37 kDa
47 kDa
Which type of bond stabilizes the structure of myoglobin?
Disulfide bond
Hydrogen bond
Ionic bond
Peptide bond
What is the heme group in myoglobin composed of?
Iron and porphyrin
Copper and porphyrin
Zinc and porphyrin
Magnesium and porphyrin
What effect does carbon monoxide (CO) have on myoglobin?
Inhibits oxygen binding
Enhances oxygen release
No effect
Increases oxygen binding
Before you leave, take our quick quiz to enhance your learning!

