What is the atomic number of Neon?
8
10
12
14
Dive into the dazzling world of Neon, the element that lights up our signs and scientific endeavors alike. This guide offers teachers a thorough understanding of Neon, including its properties, uses, and presence in everyday life. Packed with vivid examples and practical tips, you’ll find engaging ways to introduce Neon to students, ensuring your lessons shine as brightly as this noble gas. Embrace the luminescence and science of Neon in your teaching toolkit!
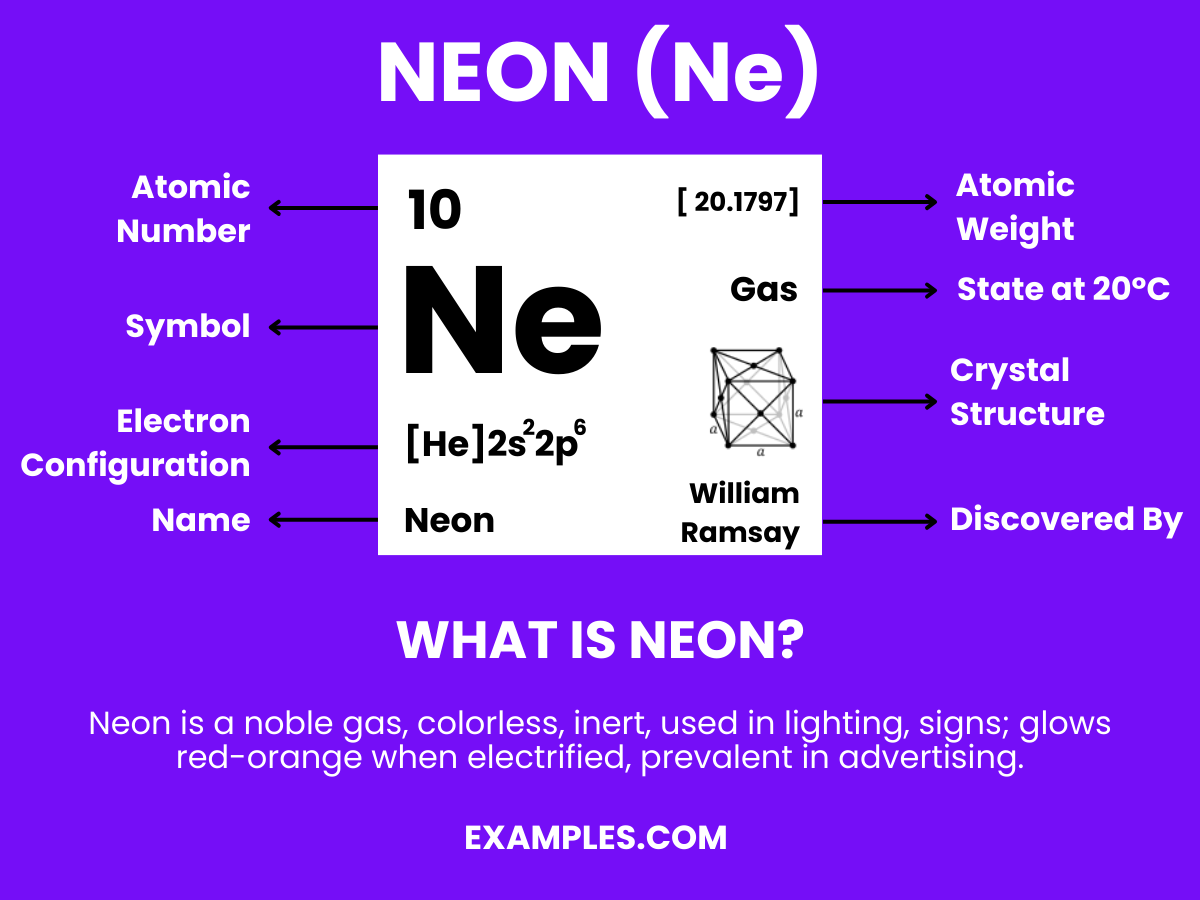
Neon is a noble gas with the chemical symbol Ne and atomic number 10, known for its distinct reddish-orange glow in neon lights. It’s inert, colorless, odorless, and tasteless in its natural state, found in small amounts in the Earth’s atmosphere. Neon has no known chemical compounds, making it a fascinating subject of study in physics and chemistry, as well as a popular element in lighting and advertising.
| Helium |
| Argon |
| Krypton |
| Xenon |
| Radon |
| Physical Property | Description |
|---|---|
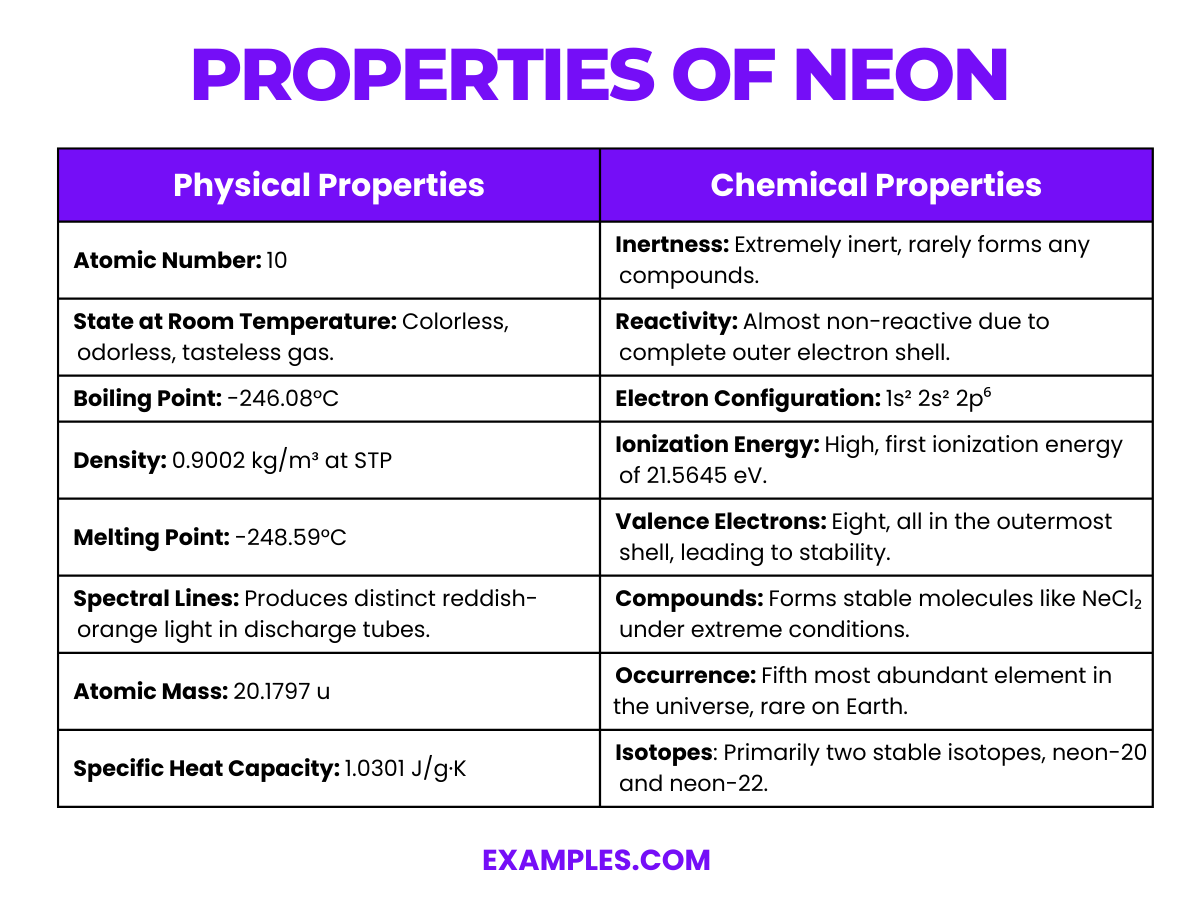
| Atomic Number | 10 |
| State at Room Temperature | Colorless, odorless, and tasteless gas. |
| Boiling Point | -246.08°C |
| Density | 0.9002 kg/m³ at STP |
| Melting Point | -248.59°C |
| Spectral Lines | Distinct reddish-orange light in discharge tubes. |
| Atomic Mass | 20.1797 u |
| Specific Heat Capacity | 1.0301 J/g·K |
| Property | Description / Value |
|---|---|
| Melting Point | -248.59°C (-415.46°F) |
| Boiling Point | -246.08°C (-410.94°F) |
| Thermal Conductivity | 0.0491 W/(m·K) |
| Specific Heat | 1.03 J/(g·K) at 298K |
| Heat of Vaporization | 1.71 kJ/mol at boiling point |
| Heat of Fusion | 0.335 kJ/mol at melting point |
| Property | Description / Value |
|---|---|
| Phase at STP | Gas |
| Density | 0.9002 g/L at 0°C, 101.325 kPa |
| Solubility in Water | 10.5 mg/L at 20°C and 1 atm |
| Color | Colorless; emits a distinct reddish-orange glow when placed in an electric field |
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Diamagnetic |
| Electrical Conductivity | Poor conductor; excels in ionized state under high voltage, leading to light emission |
| Property | Description / Value |
|---|---|
| Atomic Number | 10 |
| Atomic Mass | 20.1797 u |
| Neutron Cross Section | 0.03 barns (for ^20Ne) |
| Isotopes | ^20Ne (90.48%), ^21Ne (0.27%), ^22Ne (9.25%) |
| Radioactivity | Neon is stable with no naturally occurring radioactive isotopes. ^19Ne and ^24Ne are examples of radioactive isotopes created synthetically |
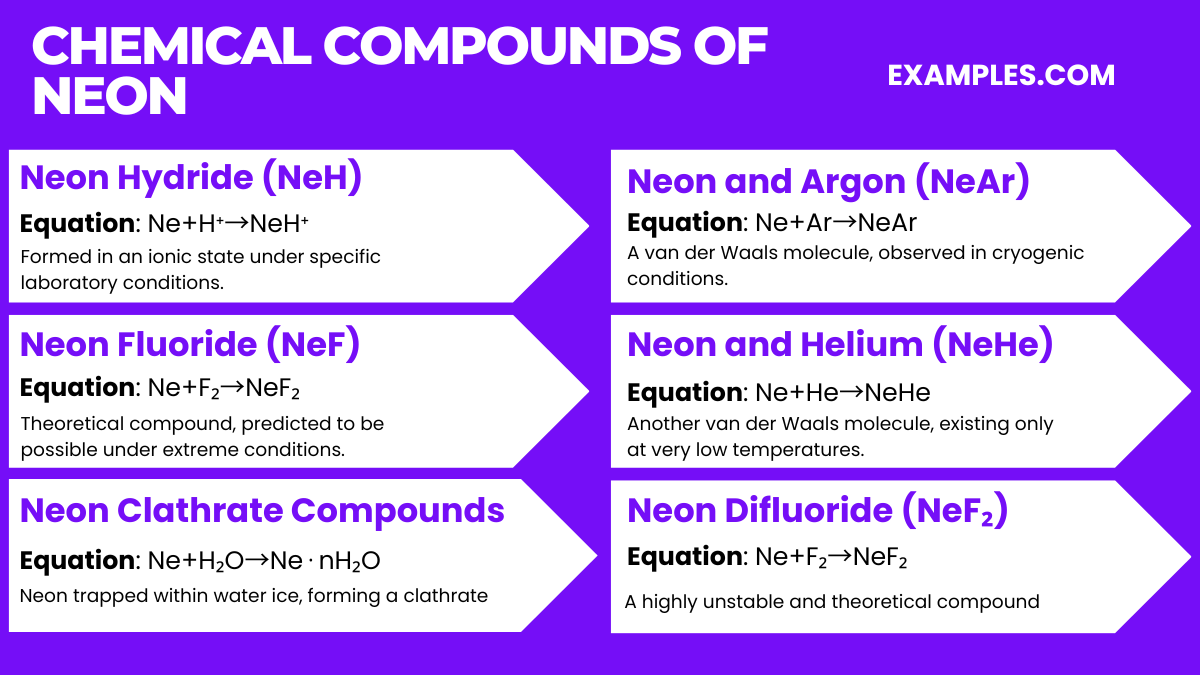
Neon, being a noble gas with a complete outer electron shell, is known for its extremely low reactivity and thus does not form stable chemical compounds under normal conditions. However, under very specific and extreme laboratory conditions, a few compounds involving neon have been theorized or experimentally observed. These compounds are not typical or widely recognized due to their unstable nature and the extreme conditions required for their formation. Here are six of these experimental or theoretical compounds:
| Isotope | Atomic Mass | Natural Abundance | Stability | Description |
|---|---|---|---|---|
| Neon-20 (²⁰Ne) | 19.9924 u | ~90.48% | Stable | The most common isotope, used in neon lights and scientific applications. |
| Neon-21 (²¹Ne) | 20.9938 u | ~0.27% | Stable | Rarer isotope, often used in tracing and in studies of extraterrestrial material. |
| Neon-22 (²²Ne) | 21.9914 u | ~9.25% | Stable | Used in nuclear studies and considered in dating applications due to its stability. |
Neon is used for its brilliant reddish-orange glow in lighting, high-voltage indicators, cryogenic refrigeration, wave meter tubes, research, and some medical imaging applications.
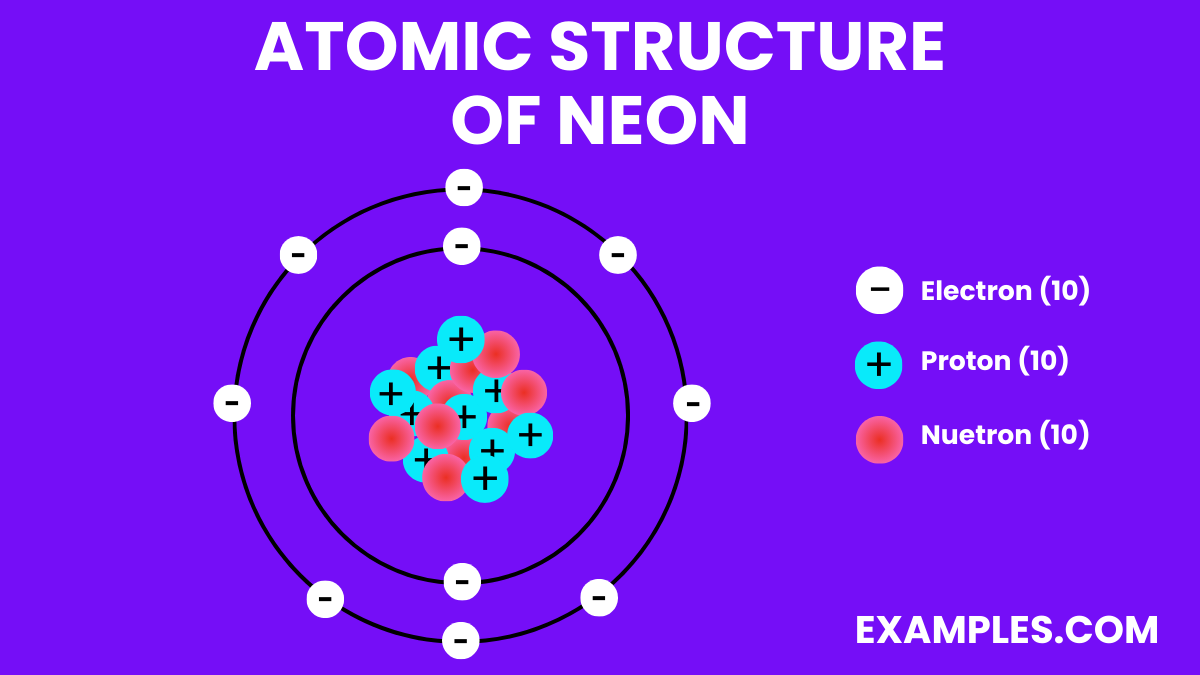
Neon has 10 protons, corresponding to its atomic number and defining its place as element number 10 on the periodic table.
In its iconic gas discharge tubes, neon emits a bright reddish-orange light when an electrical current excites its atoms.
Neon was discovered in 1898 by British chemists Sir William Ramsay and Morris W. Travers in London during experiments with liquid air.
Neon is a noble gas, known for its inertness and lack of reactivity due to a complete valence electron shell.
Neon, formed through nuclear reactions in stars, has been around since the universe began creating elements, over 4.5 billion years ago.
Neon, with its vibrant glow and inert properties, offers a spectrum of applications from lighting to cryogenics. Understanding its uses and safe handling is essential for educators and students alike. This guide aimed to enlighten your path to incorporating Neon into your curriculum, ensuring both fascination and safety in exploring this noble gas’s role in science and industry.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Neon?
8
10
12
14
Neon is a member of which group on the periodic table?
Noble gases
Alkaline earth metals
Halogens
Alkali metals
Which property of neon makes it useful in neon signs?
Colorless at room temperature
Reactivity with oxygen
Ability to emit light when electrified
Solubility in water
At what temperature does neon become a liquid?
-245.95 °C
-200.55 °C
-148.55 °C
-101.5 °C
Neon is primarily used in:
Medical imaging
Refrigeration
Lighting
Chemical synthesis
Which of the following is NOT a characteristic of neon?
High density
Low chemical reactivity
Colorless
Odorless
What percentage of the Earth\'s atmosphere is composed of neon?
18%
0.0018%
0.18%
0.018%
Neon isotopes are used in the study of:
Geological formations
Stellar formations
Ocean currents
Atmospheric phenomena
The name \"neon\" is derived from the Greek word \'neos\', which means:
Light
New
Bright
Invisible
Which is the most abundant isotope of neon?
Neon-20
Neon-21
Neon-22
Neon-24
Before you leave, take our quick quiz to enhance your learning!

