What is the atomic number of Neptunium?
91
92
93
94
Embark on a journey through the atomic realm with our comprehensive guide to Neptunium, a transuranic and enigmatic element that bridges the gap between the known and the unknown in the periodic table. sheds light on Neptunium’s discovery, its intriguing physical and chemical properties, and its role in scientific advancements and nuclear technology. Through engaging examples, we unveil the complexities and opportunities Neptunium presents to researchers, educators, and enthusiasts alike, inviting exploration into its profound implications for future innovations.
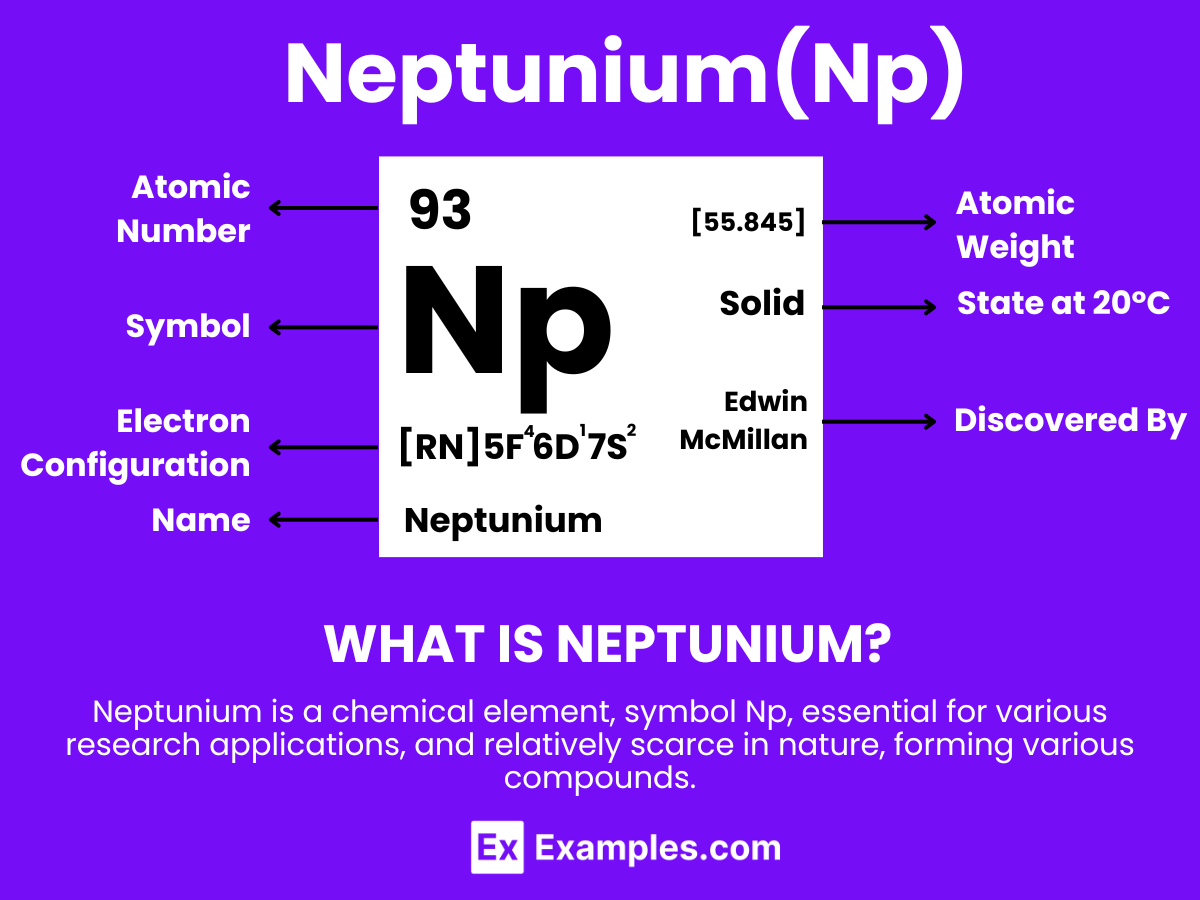
Neptunium is a dense, silvery metallic element that is recognized for its significant properties and specialized applications. With the atomic number 93, Neptunium is notable for being the first transuranic element, marking its position in the actinide series with properties that include radioactivity and the ability to form various chemical compounds. Neptunium is primarily used in research and potential applications in nuclear science, particularly in the development of neutron detection equipment and as a precursor for plutonium-238 production, which is used in radioisotope thermoelectric generators (RTGs) for spacecraft.
| Actinium | Berkelium |
| Thorium | Californium |
| Protactinium | Einsteinium |
| Uranium | Curium |
| Plutonium | Mendelevium |
| Plutonium | Nobelium |
| Americium | Lawrencium |
| Property | Value |
|---|---|
| Appearance | Silvery, metallic, with a pink tinge |
| Atomic Mass | 237 u (most stable isotope, Np-237) |
| Density | 20.45 g/cm³ at 20 °C |
| Melting Point | 644 °C |
| Boiling Point | 4174 °C |
| State at 20 °C | Solid |
| Thermal Conductivity | 6.3 W/(m·K) |
| Crystal Structure | Orthorhombic (at room temperature) |
| Electrical Resistivity | 1.220 microohm-meters (at 22 °C) |
| Magnetic Ordering | Paramagnetic at 300 K |
Neptunium is a highly reactive, radioactive metal with a variety of oxidation states, ranging from +3 to +7, which allows it to form a diverse array of compounds. Its chemical behavior is similar to other actinides.
| Property | Value |
|---|---|
| Melting Point | 644 °C |
| Boiling Point | 4,017 °C |
| Heat of Fusion | 3.20 kJ/mol |
| Heat of Vaporization | 336 kJ/mol |
| Specific Heat Capacity | 29.46 J/(mol·K) (at 25 °C) |
| Property | Value |
|---|---|
| Density (at room temperature) | 20.45 g/cm³ |
| Crystal Structure | Orthorhombic (at room temperature) |
| Hardness | Vickers: 320 HV |
| Thermal Conductivity | 6.3 W/(m·K) |
| Electrical Resistivity | 1.220 μΩ·m (at 0 °C) |
| Property | Value |
|---|---|
| Magnetic Ordering | Paramagnetic |
| Electrical Conductivity | Metallic conductor |
| Magnetic Susceptibility | +2800.0·10⁻⁶ cm³/mol (at 298 K) |
| Property | Value |
|---|---|
| Primary Isotopes | Neptunium-237 |
| Half-life of Np-237 | 2.14 million years |
| Decay Mode of Np-237 | Alpha decay to Protactinium-233 |
| Neutron Cross Section (Np-237) | 175 barns for thermal neutrons |
| Fission Products | Various, depending on neutron capture and fission process |
| Isotope | Mass Number | Half-life | Decay Mode |
|---|---|---|---|
| Np-237 | 237 | 2.14 million years | Alpha decay |
| Np-236 | 236 | 154,000 years | Alpha decay, Neutron emission |
| Np-235 | 235 | 396.1 days | Beta decay |
| Np-239 | 239 | 2.356 days | Beta decay |
Neptunium, a synthetic element with the atomic number 93, is primarily produced in nuclear reactors. The most common isotope of neptunium, neptunium-237 (Np-237), is created through a series of nuclear reactions that begin with the absorption of a neutron by uranium-235 (U-235), one of the common fuels used in nuclear reactors. This process involves several beta decays, where a neutron within the nucleus of an atom decays into a proton, releasing electrons and antineutrinos in the process.
The production of neptunium in a nuclear reactor typically follows this sequence:
Neptunium can also be produced by bombarding uranium-238 (U-238) with neutrons in a reactor, which transforms it into plutonium-239 (Pu-239) through a series of neutron captures and beta decays. Pu-239 can then capture another neutron and undergo beta decay to become Np-239, which has a relatively short half-life and decays into plutonium-239, a process used for producing plutonium in breeder reactors.
Neptunium’s applications are limited due to its radioactivity and the complexity of handling it safely. However, it has several important uses:
Neptunium, with its unique position on the periodic table, offers a fascinating glimpse into the complex world of transuranic elements. Despite its relative obscurity and challenges in handling due to its radioactivity, its potential in scientific research and nuclear applications cannot be understated. The exploration of Neptunium not only deepens our understanding of atomic structure and nuclear physics but also paves the way for advancements in energy production and material science.

Embark on a journey through the atomic realm with our comprehensive guide to Neptunium, a transuranic and enigmatic element that bridges the gap between the known and the unknown in the periodic table. sheds light on Neptunium’s discovery, its intriguing physical and chemical properties, and its role in scientific advancements and nuclear technology. Through engaging examples, we unveil the complexities and opportunities Neptunium presents to researchers, educators, and enthusiasts alike, inviting exploration into its profound implications for future innovations.
Neptunium is a dense, silvery metallic element that is recognized for its significant properties and specialized applications. With the atomic number 93, Neptunium is notable for being the first transuranic element, marking its position in the actinide series with properties that include radioactivity and the ability to form various chemical compounds. Neptunium is primarily used in research and potential applications in nuclear science, particularly in the development of neutron detection equipment and as a precursor for plutonium-238 production, which is used in radioisotope thermoelectric generators (RTGs) for spacecraft.
Formula: Np
Composition: Consists of a single neptunium atom.
Bond Type: In its elemental form, neptunium does not have bonds as it is a pure element. However, neptunium can form covalent or ionic bonds when reacting with other elements.
Molecular Structure: As a pure element, neptunium does not form a molecular structure like compounds. At room temperature, neptunium is in a metallic state with an orthorhombic crystalline structure, transitioning to a face-centered cubic (fcc) structure at higher temperatures.
Electron Sharing: In compounds, neptunium typically shares electrons covalently or transfers electrons ionically, depending on the nature of the other element(s) it is bonding with.
Significance: Neptunium is significant for its role in nuclear reactors and potential use in nuclear weapons. Its isotopes, such as Neptunium-237, are used in neutron detection and as precursors for the production of Plutonium-238, used in radioisotope thermoelectric generators (RTGs) in spacecraft.
Role in Chemistry: Neptunium plays a crucial role in nuclear chemistry and reactor technology. Its ability to form various compounds, including oxides and halides, is essential for nuclear fuel cycles and research into transuranic waste management and actinide behavior, marking it as a vital material in nuclear science and technology.
Primary Configuration: Neptunium’s electron configuration is [Rn] 5f⁴ 6d¹ 7s².
Energy Levels: This configuration indicates Neptunium has electrons in seven energy levels, with the outermost shell containing two electrons.
Atomic Number: Neptunium has an atomic number of 93, denoting the number of protons in its nucleus.
Atomic Mass: The atomic mass of Neptunium is approximately 237 u (atomic mass units), reflecting the sum of protons and neutrons in its nucleus.
Radioactive Nature: All isotopes of Neptunium are radioactive, with Neptunium-237 being the most stable and widely studied.
Half-life: Neptunium-237 has a half-life of about 2.14 million years, indicating its rate of decay over time.
Oxidation States: Neptunium exhibits multiple oxidation states, with +3, +4, +5, and +6 being the most common in chemical compounds.
Reactivity: It reacts with oxygen, acids, and compounds, forming various Neptunium compounds depending on its oxidation state.
Appearance: Pure Neptunium metal has a silvery appearance, but it tarnishes when exposed to air.
Phase at Room Temperature: It is solid under standard conditions of temperature and pressure.
Breeder Reactors: Neptunium-237’s ability to absorb neutrons and breed plutonium-238 makes it significant in nuclear reactors and space exploration as a potential fuel source.
Radiation Precautions: Handling Neptunium requires strict safety protocols to protect against its alpha radiation.
Environmental Impact: Understanding and mitigating the environmental impact of Neptunium, especially from nuclear waste, is crucial for its safe use
Property | Value |
|---|---|
Appearance | Silvery, metallic, with a pink tinge |
Atomic Mass | 237 u (most stable isotope, Np-237) |
Density | 20.45 g/cm³ at 20 °C |
Melting Point | 644 °C |
Boiling Point | 4174 °C |
State at 20 °C | Solid |
Thermal Conductivity | 6.3 W/(m·K) |
Crystal Structure | Orthorhombic (at room temperature) |
Electrical Resistivity | 1.220 microohm-meters (at 22 °C) |
Magnetic Ordering | Paramagnetic at 300 K |
Neptunium is a highly reactive, radioactive metal with a variety of oxidation states, ranging from +3 to +7, which allows it to form a diverse array of compounds. Its chemical behavior is similar to other actinides.
Oxidation States: Neptunium easily shifts between oxidation states in solutions, affecting its chemical reactions and compound formation.
Equation Example: NpO₂⁺ (oxidation state +5) can be both oxidized to NpO₂²⁺ (oxidation state +6) and reduced to Np⁴⁺ (oxidation state +4).
Reaction with Water: Neptunium reacts with water, forming oxides and hydroxides along with releasing hydrogen gas.
Equation: Np + 2 H₂O → NpO₂ + 2 H₂↑
Reaction with Acids: Neptunium dissolves in acidic solutions, forming various neptunium ions depending on the acid and its concentration.
Equation: Np + 4 HNO₃ → Np(NO₃)₄ + 2 H₂O
Formation of Halides: Neptunium reacts with halogens to form halides, such as neptunium fluoride (NpF₆), which is used in uranium enrichment processes.
Equation: Np + 3 F₂ → NpF₆
Complexation and Coordination: Neptunium forms complex ions and coordination compounds, showing its ability to bond with various ligands.
Equation Example: NpO₂⁺ + 2 OH⁻ → [NpO₂(OH)₂]⁻
Property | Value |
|---|---|
Melting Point | 644 °C |
Boiling Point | 4,017 °C |
Heat of Fusion | 3.20 kJ/mol |
Heat of Vaporization | 336 kJ/mol |
Specific Heat Capacity | 29.46 J/(mol·K) (at 25 °C) |
Property | Value |
|---|---|
Density (at room temperature) | 20.45 g/cm³ |
Crystal Structure | Orthorhombic (at room temperature) |
Hardness | Vickers: 320 HV |
Thermal Conductivity | 6.3 W/(m·K) |
Electrical Resistivity | 1.220 μΩ·m (at 0 °C) |
Property | Value |
|---|---|
Magnetic Ordering | Paramagnetic |
Electrical Conductivity | Metallic conductor |
Magnetic Susceptibility | +2800.0·10⁻⁶ cm³/mol (at 298 K) |
Property | Value |
|---|---|
Primary Isotopes | Neptunium-237 |
Half-life of Np-237 | 2.14 million years |
Decay Mode of Np-237 | Alpha decay to Protactinium-233 |
Neutron Cross Section (Np-237) | 175 barns for thermal neutrons |
Fission Products | Various, depending on neutron capture and fission process |
Neptunium Oxide (NpO₂): A solid brown compound used in nuclear fuel processing and waste management. Represents neptunium in the +4 oxidation state.
Equation: Np+12O₂→NpO₂
Neptunium Dioxide (NpO₂): Black, crystalline solid acting as an important component in nuclear reactors, primarily in the +4 oxidation state.
Equation: 2Np+O₂→2NpO₂(Note: This reaction describes the formation of neptunium dioxide, which is similar to the oxide but emphasizes the solid state nature and its use.)
Neptunium Trioxide (Np₂O₃): A brown-black solid that is less stable and common, showcasing neptunium in the +3 oxidation state.
Equation: 4Np+32O₂→2Np₂O₃
Neptunium Tetrafluoride (NpF₄): Green solid used in chemical separations, representing neptunium in the +4 oxidation state.
Equation: Np+2F₂→NpF₄
Neptunium Hexafluoride (NpF₆): Volatile, yellow compound important for uranium enrichment processes, in the +6 oxidation state.
Equation: Np+3F₂→NpF₆
Neptunium Pentoxide (Np₂O₅): An unstable, yellowish compound, often used in studies of neptunium’s chemistry, mainly in the +5 oxidation state.
Equation: 2Np+5₂O₂→Np2O₅
Isotope | Mass Number | Half-life | Decay Mode |
|---|---|---|---|
Np-237 | 237 | 2.14 million years | Alpha decay |
Np-236 | 236 | 154,000 years | Alpha decay, Neutron emission |
Np-235 | 235 | 396.1 days | Beta decay |
Np-239 | 239 | 2.356 days | Beta decay |
Np-237: This is the most commonly encountered isotope, known for its relatively long half-life. It’s a byproduct of nuclear reactors and nuclear weapons tests.
Np-236: Has a significant half-life and can undergo both alpha decay and neutron emission. It’s of interest for nuclear science research.
Np-235: With a much shorter half-life, it decays by beta emission to plutonium-235.
Np-239: Decays quickly through beta emission to plutonium-239, which is a key fissile material for nuclear reactors and nuclear weapons.
Nuclear Reactors: Neptunium-237 can be used in the production of plutonium-238, which is utilized in radioisotope thermoelectric generators (RTGs) for spacecraft and remote terrestrial applications due to its high power density and long half-life.
Research: Due to its various isotopes and their decay modes, neptunium is of interest in nuclear physics research, including studies on nuclear reactions and nuclear structure.
Nuclear Material Tracking: The distinct gamma-ray signatures of neptunium isotopes are used in nuclear forensics to identify and track the origin and movement of nuclear materials.
Potential Use in Nuclear Weapons: While not a primary material, neptunium-237’s potential for use in nuclear weapons has been studied due to its ability to be transformed into plutonium-238 upon neutron capture.
Radiation Therapy Development: Neptunium-237’s radioactive properties and decay process are studied for potential applications in developing new methods for targeted radiation therapy in cancer treatment
Neptunium, a synthetic element with the atomic number 93, is primarily produced in nuclear reactors. The most common isotope of neptunium, neptunium-237 (Np-237), is created through a series of nuclear reactions that begin with the absorption of a neutron by uranium-235 (U-235), one of the common fuels used in nuclear reactors. This process involves several beta decays, where a neutron within the nucleus of an atom decays into a proton, releasing electrons and antineutrinos in the process.
The production of neptunium in a nuclear reactor typically follows this sequence:
Uranium-235 captures a neutron, becoming uranium-236 (U-236).
U-236 undergoes beta decay to form neptunium-237 (Np-237).
Neptunium can also be produced by bombarding uranium-238 (U-238) with neutrons in a reactor, which transforms it into plutonium-239 (Pu-239) through a series of neutron captures and beta decays. Pu-239 can then capture another neutron and undergo beta decay to become Np-239, which has a relatively short half-life and decays into plutonium-239, a process used for producing plutonium in breeder reactors.
Neptunium’s applications are limited due to its radioactivity and the complexity of handling it safely. However, it has several important uses:
Neptunium-237 as a Precursor for Plutonium-238 Production: Np-237 is used to produce plutonium-238 (Pu-238) through neutron bombardment in a nuclear reactor. Pu-238 has vital applications, most notably as a heat source in radioisotope thermoelectric generators (RTGs) used in spacecraft. These RTGs have powered missions to Mars, the outer planets, and beyond, providing reliable electricity for decades without sunlight.
Research and Development: Neptunium plays a role in nuclear research, particularly in studies related to its behavior in nuclear waste and its potential for use in nuclear weapons. Understanding the long-term behavior of neptunium is crucial for the development of effective strategies for the management and disposal of radioactive waste.
Potential Use in Nuclear Batteries: While not widely implemented, there is research into using neptunium in nuclear batteries, where its radioactive decay could provide a long-lasting energy source for devices that require low power over extended periods.
Neptunium, with its unique position on the periodic table, offers a fascinating glimpse into the complex world of transuranic elements. Despite its relative obscurity and challenges in handling due to its radioactivity, its potential in scientific research and nuclear applications cannot be understated. The exploration of Neptunium not only deepens our understanding of atomic structure and nuclear physics but also paves the way for advancements in energy production and material science.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons (93)
Neutrons (144)
Protons (93)
What is the atomic number of Neptunium?
91
92
93
94
What is the chemical symbol for Neptunium?
Np
Ne
Nu
Nt
Neptunium belongs to which series in the periodic table?
Lanthanides
Actinides
Transition metals
Halogens
What is the most stable isotope of Neptunium?
Neptunium-235
Neptunium-236
Neptunium-237
Neptunium-238
Neptunium was the first synthetic element discovered after which element?
Uranium
Thorium
Plutonium
Curium
In which year was Neptunium first synthesized?
1934
1936
1940
1942
Which element is formed as a decay product of Neptunium-237?
Uranium-235
Plutonium-237
Thorium-232
Protactinium-233
Neptunium is typically found in which oxidation state in aqueous solutions?
+2
+3
+4
+5
Which of the following is a common application of Neptunium?
Fuel for nuclear reactors
Fuel for nuclear reactors
Spacecraft shielding
Dental fillings
What is the primary source of Neptunium?
Natural uranium deposits
Synthesis in particle accelerators
Byproduct of nuclear reactors
Extraction from seawater
Before you leave, take our quick quiz to enhance your learning!

