What is the atomic number of Nickel?
26
27
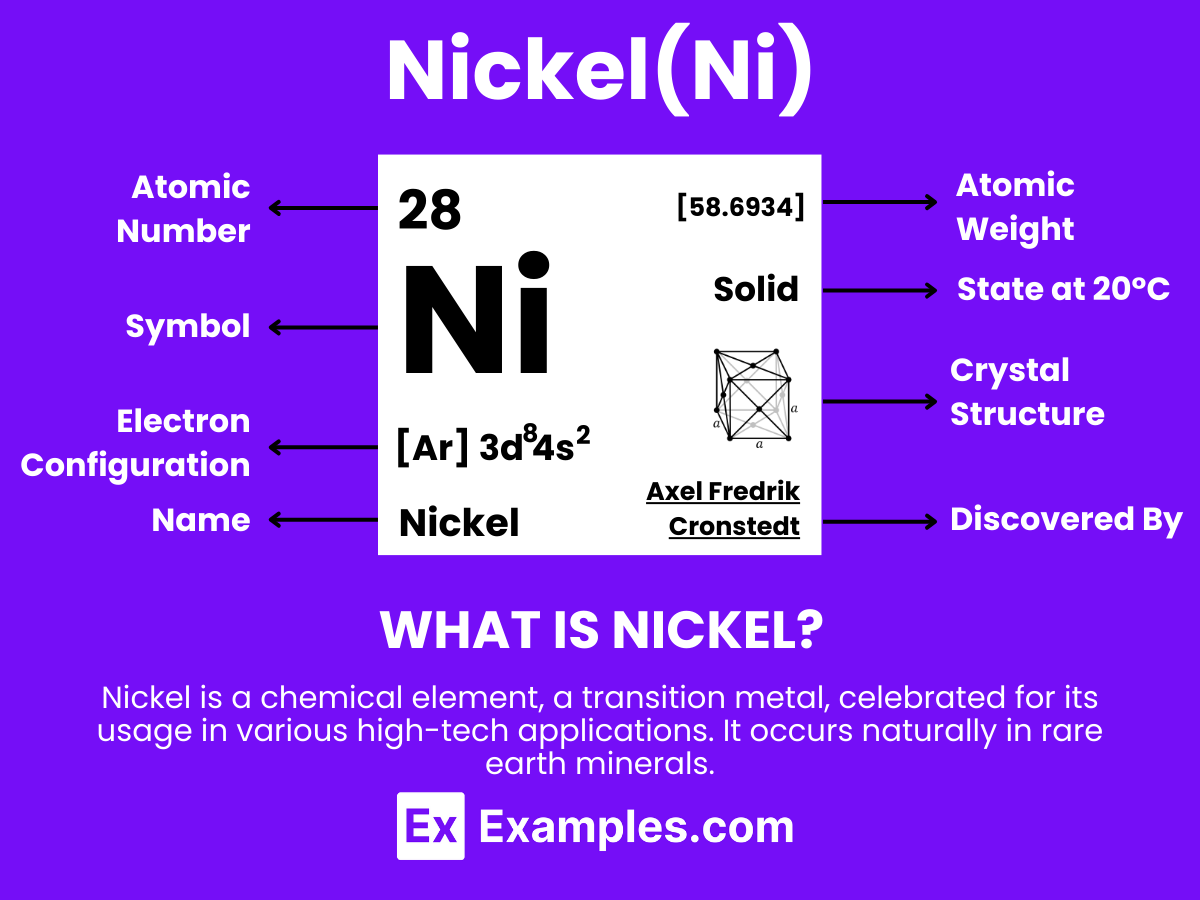
28
29
Discover the dynamic world of Nickel, a versatile element pivotal to our modern lifestyle. From its role in crafting durable stainless steel to powering rechargeable batteries, nickel’s unique properties make it indispensable across industries. This guide delves into nickel’s journey from extraction to application, showcasing examples that highlight its significance in corrosion resistance, energy storage, and green technologies. Embark on an exploration of how nickel’s applications are shaping a sustainable future, illustrating its critical role in innovation and environmental stewardship
Nickel is a hard, silvery-white metallic element known for its outstanding properties and broad range of applications, with the atomic number 28. It is distinguished by its excellent resistance to corrosion and oxidation, making it an ideal material for use in extreme conditions. Nickel does not occur freely in nature but is primarily found in combination with sulfur, iron, and other metals in various ores from which it is extracted. This element is extensively utilized across numerous sectors, particularly in the production of stainless steel and other corrosion-resistant alloys used in construction, the automotive industry, and for making coins. Additionally, nickel finds important applications in the chemical industry as a catalyst for hydrogenation, in battery production, particularly in rechargeable nickel-cadmium batteries and nickel-metal hydride batteries used in portable devices and electric vehicles, and in electronics for creating durable and reliable components.
Below is a table detailing the physical properties of nickel, a highly versatile and widely utilized metallic element:
| Property | Value |
|---|---|
| Appearance | Lustrous, metallic, silver-white |
| Atomic Number | 28 |
| Atomic Mass | 58.6934 u |
| Density at 20°C | 8.908 g/cm³ |
| Melting Point | 1455°C (2651°F) |
| Boiling Point | 2913°C (5275°F) |
| State at 20°C | Solid |
| Electrical Conductivity | 14.3 × 10^6 S/m |
| Thermal Conductivity | 90.9 W/(m·K) |
| Heat of Fusion | 17.48 kJ/mol |
| Heat of Vaporization | 377.5 kJ/mol |
| Specific Heat Capacity | 26.07 J/(mol·K) |
Thermal Stability: Nickel compounds are thermally stable, suitable for high-temperature applications.
Catalytic Properties: Acts as a catalyst in hydrogenation processes, aiding in the conversion of unsaturated compounds.
Magnetic Properties: Exhibits ferromagnetic properties, useful in magnetic materials.
Environmental and Biological Role: Essential in small quantities for plant growth but toxic in high concentrations.
| Property | Value |
|---|---|
| Melting Point | 1455°C (2651°F) |
| Boiling Point | 2913°C (5275°F) |
| Heat of Fusion | 17.48 kJ/mol |
| Heat of Vaporization | 377.5 kJ/mol |
| Specific Heat Capacity | 26.07 J/(mol·K) |
| Thermal Conductivity | 90.9 W/(m·K) |
| Thermal Expansion | 13.4 µm/(m·K) at 25°C |
| Property | Value |
|---|---|
| Atomic Mass | 58.6934 u |
| Density | 8.908 g/cm³ at 20°C |
| Mohs Hardness | 4 |
| Young’s Modulus | 200 GPa |
| Bulk Modulus | 180 GPa |
| Poisson’s Ratio | 0.31 |
| Property | Value |
|---|---|
| Electrical Resistivity | 6.99 µΩ·m at 20°C |
| Magnetic Ordering | Ferromagnetic |
| Curie Temperature | 358°C (676°F) |
| Magnetic Susceptibility | High |
| Property | Value |
|---|---|
| Isotopes | Mainly ^58Ni, ^60Ni, ^61Ni, ^62Ni, ^64Ni |
| Atomic Number | 28 |
| Atomic Weight | 58.6934 |
| Stable Isotopes | ^58Ni (most abundant) |
| Neutron Cross Section | 4.5 barns (for ^58Ni) |
| Neutron Mass Absorption | 0.0012 |
The preparation of nickel involves several complex processes designed to extract and purify nickel from its ores. Nickel is predominantly obtained from two types of ore deposits: laterites and sulfides. Each type of ore requires a different extraction and processing technique. Here’s an overview of the main methods used in the preparation of nickel:
| Isotope | Mass Number | Natural Abundance (%) | Half-Life | Notes |
|---|---|---|---|---|
| Ni-58 | 58 | 68.077% | Stable | Most abundant isotope |
| Ni-60 | 60 | 26.223% | Stable | |
| Ni-61 | 61 | 1.140% | Stable | |
| Ni-62 | 62 | 3.635% | Stable | Has the highest binding energy per nucleon of any isotope |
| Ni-64 | 64 | 0.925% | Stable | |
| Ni-59 | 59 | Synthetic | 76,000 years | Used in nuclear reactors |
| Ni-63 | 63 | Synthetic | 100.1 years | Used in betavoltaic devices |
Nickel is utilized across various industries, highlighting its versatility and significance in both everyday and specialized applications.
nickel has illuminated its indispensable role across numerous industries, from high-performance alloys to green energy solutions. The detailed table we’ve presented showcases nickel’s unique physical and chemical properties, underlining its versatility and durability. This insight into nickel’s preparation and applications emphasizes its pivotal contribution to technological advancements and sustainable development, making it a key element in our pursuit of innovation

Discover the dynamic world of Nickel, a versatile element pivotal to our modern lifestyle. From its role in crafting durable stainless steel to powering rechargeable batteries, nickel’s unique properties make it indispensable across industries. This guide delves into nickel’s journey from extraction to application, showcasing examples that highlight its significance in corrosion resistance, energy storage, and green technologies. Embark on an exploration of how nickel’s applications are shaping a sustainable future, illustrating its critical role in innovation and environmental stewardship
Nickel is a hard, silvery-white metallic element known for its outstanding properties and broad range of applications, with the atomic number 28. It is distinguished by its excellent resistance to corrosion and oxidation, making it an ideal material for use in extreme conditions. Nickel does not occur freely in nature but is primarily found in combination with sulfur, iron, and other metals in various ores from which it is extracted. This element is extensively utilized across numerous sectors, particularly in the production of stainless steel and other corrosion-resistant alloys used in construction, the automotive industry, and for making coins. Additionally, nickel finds important applications in the chemical industry as a catalyst for hydrogenation, in battery production, particularly in rechargeable nickel-cadmium batteries and nickel-metal hydride batteries used in portable devices and electric vehicles, and in electronics for creating durable and reliable components.
Formula: Ni
Nickel is represented by the chemical symbol Ni, denoting a single atom of nickel in its elemental form.
Composition: Nickel consists of a single nickel atom, classifying it as a pure element. Unlike chemical compounds, it does not combine with itself to form a molecular structure.
Bond Type: In its elemental state, nickel does not form chemical bonds since it exists as a pure metal. However, nickel can participate in both covalent and ionic bonding when it reacts with other elements, showcasing its chemical versatility.
Molecular Structure: As a pure element, nickel does not have a molecular structure similar to compounds like H₂O. It exists in a metallic state, adopting a face-centered cubic (fcc) crystalline structure at room temperature, which is typical for many metals.
Electron Sharing: In the formation of compounds, nickel commonly shares electrons covalently or engages in ionic bonding, depending on the nature of the elements it interacts with. This electron sharing capability underlines nickel’s reactivity and ability to form a wide range of compounds.
Significance: Nickel is renowned for its corrosion resistance and exceptional strength at high temperatures, making it indispensable in stainless steel and other corrosion-resistant alloys. Its catalytic properties are also crucial in hydrogenation reactions and in the synthesis of various chemicals.
Role in Chemistry: Nickel plays a pivotal role in catalysis, notably in the hydrogenation of unsaturated compounds in organic chemistry. Its compounds are vital in producing advanced materials, batteries, and for promoting sustainable chemical processes. This underscores nickel’s importance in modern technological and chemical advancements, similar to the role of ruthenium but with its distinct applications and properties.
Protons: 28, defining its chemical identity.
Neutrons: Varies, with nickel-58 being the most common isotope.
Electrons: 28, orbiting in shells, crucial for chemical reactions.
Configuration: [Ar] 3d⁸4s², indicating its status as a transition metal.
Importance: Influences bonding, reactivity, and alloy formation.
Atomic Radius: Approx. 124 picometers, affecting density and structure.
Ionic Radius: Changes with ion charge, impacting compound formation.
Ferromagnetic: One of the few elements with this property at room temperature.
Utilization: Used in magnets and electronic devices due to electron spin alignment
Below is a table detailing the physical properties of nickel, a highly versatile and widely utilized metallic element:
Property | Value |
|---|---|
Appearance | Lustrous, metallic, silver-white |
Atomic Number | 28 |
Atomic Mass | 58.6934 u |
Density at 20°C | 8.908 g/cm³ |
Melting Point | 1455°C (2651°F) |
Boiling Point | 2913°C (5275°F) |
State at 20°C | Solid |
Electrical Conductivity | 14.3 × 10^6 S/m |
Thermal Conductivity | 90.9 W/(m·K) |
Heat of Fusion | 17.48 kJ/mol |
Heat of Vaporization | 377.5 kJ/mol |
Specific Heat Capacity | 26.07 J/(mol·K) |
Nickel(II) (Ni²⁺): Most common and stable, found in many nickel compounds.
Nickel(III) (Ni³⁺): Less common, known for oxidizing properties.
With Oxygen: Forms nickel(II) oxide (NiO).
With Acids: Produces nickel(II) salts and hydrogen gas (e.g., Ni + 2HCl → NiCl₂ + H₂).
With Water: Nickel is corrosion-resistant and does not react under normal conditions.
Thermal Stability: Nickel compounds are thermally stable, suitable for high-temperature applications.
Catalytic Properties: Acts as a catalyst in hydrogenation processes, aiding in the conversion of unsaturated compounds.
Magnetic Properties: Exhibits ferromagnetic properties, useful in magnetic materials.
Environmental and Biological Role: Essential in small quantities for plant growth but toxic in high concentrations.
Property | Value |
|---|---|
Melting Point | 1455°C (2651°F) |
Boiling Point | 2913°C (5275°F) |
Heat of Fusion | 17.48 kJ/mol |
Heat of Vaporization | 377.5 kJ/mol |
Specific Heat Capacity | 26.07 J/(mol·K) |
Thermal Conductivity | 90.9 W/(m·K) |
Thermal Expansion | 13.4 µm/(m·K) at 25°C |
Property | Value |
|---|---|
Atomic Mass | 58.6934 u |
Density | 8.908 g/cm³ at 20°C |
Mohs Hardness | 4 |
Young’s Modulus | 200 GPa |
Bulk Modulus | 180 GPa |
Poisson’s Ratio | 0.31 |
Property | Value |
|---|---|
Electrical Resistivity | 6.99 µΩ·m at 20°C |
Magnetic Ordering | Ferromagnetic |
Curie Temperature | 358°C (676°F) |
Magnetic Susceptibility | High |
Property | Value |
|---|---|
Isotopes | Mainly ^58Ni, ^60Ni, ^61Ni, ^62Ni, ^64Ni |
Atomic Number | 28 |
Atomic Weight | 58.6934 |
Stable Isotopes | ^58Ni (most abundant) |
Neutron Cross Section | 4.5 barns (for ^58Ni) |
Neutron Mass Absorption | 0.0012 |
The preparation of nickel involves several complex processes designed to extract and purify nickel from its ores. Nickel is predominantly obtained from two types of ore deposits: laterites and sulfides. Each type of ore requires a different extraction and processing technique. Here’s an overview of the main methods used in the preparation of nickel:
Sulfide Ores Processing:
Froth Flotation: Sulfide ores are crushed and ground to liberate the nickel-containing sulfide minerals from the waste rock. The ground ore is then subjected to froth flotation to separate the sulfide minerals from the gangue.
Smelting: The concentrated ore is then smelted in a flash furnace where it reacts with oxygen to produce a nickel matte, containing 10-20% nickel.
Converter Process: The nickel matte is further processed in a converter to remove sulfur, resulting in a high-purity nickel sulfide.
Refining: The nickel sulfide is then refined to produce pure nickel metal through processes such as the Mond process, where it is reacted with carbon monoxide to form nickel carbonyl gas, which is decomposed at high temperatures to yield high-purity nickel.
Laterite Ores Processing:
Pressure Acid Leach (PAL): Laterite ores are subjected to high-pressure acid leaching to dissolve nickel and cobalt, leaving behind the waste material.
Purification and Recovery: The leach solution is then purified, and nickel is recovered via precipitation, solvent extraction, or electrowinning. This process typically yields nickel as a hydroxide or sulfate, which can be further processed into pure nickel metal.
Electrorefining: Pure nickel metal can also be produced by electrorefining nickel from the purified aqueous nickel solutions obtained from sulfide or laterite ores. In this process, nickel is electroplated onto a cathode from nickel sulfate or chloride solutions in electrolytic cells
Nickel(II) Chloride (NiCl₂)
A versatile compound used in electroplating and as a catalyst in organic synthesis.
Equation: 2Ni+Cl₂→NiCl₂
Nickel(II) Sulfate (NiSO₄)
Important for nickel plating and as a precursor for various nickel compounds.
Equation: 2↑Ni+H₂SO₄→NiSO₄+H₂↑
Nickel(II) Oxide (NiO)
Used in ceramics and as a cathode material in batteries.
Equation: 2Ni+O₂→2NiO
Nickel(II) Carbonate (NiCO₃)
Serves as a precursor to nickel(II) oxide in the production of catalysts and pigments.
Equation: NiCl₂+Na₂CO₃→NiCO₃↓+2NaCl
Nickel(II) Hydroxide (Ni(OH)₂)
A component in rechargeable battery electrodes, particularly in nickel-cadmium batteries.
Equation: NiCl₂+2NaOH→Ni(OH)₂↓+2NaCl
Nickel(II) Nitrate (Ni(NO₃)₂)
Employed in the production of nickel catalysts and for coloring ceramics.
Equation: 2↑Ni+2HNO₃→Ni(NO3)₂+H₂↑
Isotope | Mass Number | Natural Abundance (%) | Half-Life | Notes |
|---|---|---|---|---|
Ni-58 | 58 | 68.077% | Stable | Most abundant isotope |
Ni-60 | 60 | 26.223% | Stable | |
Ni-61 | 61 | 1.140% | Stable | |
Ni-62 | 62 | 3.635% | Stable | Has the highest binding energy per nucleon of any isotope |
Ni-64 | 64 | 0.925% | Stable | |
Ni-59 | 59 | Synthetic | 76,000 years | Used in nuclear reactors |
Ni-63 | 63 | Synthetic | 100.1 years | Used in betavoltaic devices |
Nickel is utilized across various industries, highlighting its versatility and significance in both everyday and specialized applications.
Stainless Steel Production: Nickel is a key component in stainless steel, enhancing its corrosion resistance and strength.
Alloys: Forms various alloys used in jet engines, gas turbines, and special engineering materials for high-temperature and corrosion-resistant applications.
Batteries: Critical in rechargeable battery systems, including nickel-metal hydride (NiMH) batteries for electronic devices and electric vehicles.
Catalysis: Used as a catalyst in hydrogenation processes in the chemical industry, improving the efficiency of chemical reactions.
Electronics: Nickel is used in the production of electronic components, including resistors and capacitors, due to its electrical conductivity.
Coinage: Nickel has historically been used in the minting of coins, providing durability and resistance to corrosion.
Magnetic Materials: Utilized in the production of ferromagnetic materials for motors, generators, and magnetic storage devices
Ore Types: Primarily pentlandite in sulfide ores and laterites.
Extraction: Sulfide ores processed by flotation; laterites by pressure acid leaching.
Pyrometallurgical Process: For sulfide ores, involving smelting and converting to produce matte.
Hydrometallurgical Process: For laterites, includes leaching, solvent extraction, and electrowinning.
Electrorefining: Produces high-purity nickel from matte.
Mond Process: Converts nickel oxides to pure nickel metal through carbon monoxide gas.
Stainless Steel: Enhances corrosion resistance and mechanical properties.
Superalloys: Critical for high-temperature applications in aerospace and power generation.
Rechargeable Batteries: Nickel-metal hydride (NiMH) and lithium-ion batteries.
Hydrogenation Reactions: Nickel catalysts are used in the food industry and hydrogen production.
Plating: Nickel plating provides wear resistance and electrical conductivity.
Currency Production: Nickel’s durability and resistance to corrosion make it ideal for coins
nickel has illuminated its indispensable role across numerous industries, from high-performance alloys to green energy solutions. The detailed table we’ve presented showcases nickel’s unique physical and chemical properties, underlining its versatility and durability. This insight into nickel’s preparation and applications emphasizes its pivotal contribution to technological advancements and sustainable development, making it a key element in our pursuit of innovation
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Electrons
Neutrons
Protons
What is the atomic number of Nickel?
26
27
28
29
Nickel is primarily obtained from which mineral?
Hematite
Bauxite
Chalcopyrite
Pentlandite
What is the symbol for Nickel on the periodic table?
Ni
Nk
Nc
Nl
Nickel belongs to which group in the periodic table?
Group 9
Group 10
Group 11
Group 12
What is the common oxidation state of Nickel?
+1
+2
+3
+4
Which of the following is a common use for Nickel?
Construction of buildings
Manufacturing of batteries
Food preservation
Production of fertilizers
Which process is commonly used to extract Nickel from its ores?
Froth flotation
Smelting
Bayer process
Electrorefining
What is the electron configuration of Nickel?
[Ar] 3d8 4s2
[Ar] 3d9 4s1
[Ar] 3d10
[Ar] 3d6 4s2
Which of the following properties is NOT associated with Nickel?
High corrosion resistance
Ferromagnetic
High electrical conductivity
Low melting point
Nickel can form alloys with many metals. Which of the following is NOT a Nickel alloy?
Monel
Invar
Bronze
Hastelloy
Before you leave, take our quick quiz to enhance your learning!

