What is the chemical formula of nitrogen dioxide?
NO
NO₂
N₂O
N₂O₄


Nitrogen dioxide, often symbolized as NO₂,s a fascinating molecular compound in the field of chemistry. Nitrogen dioxide is a gas that’s both intriguing and vital to understand, especially for young science enthusiasts. Picture this: a reddish-brown gas with a sharp, acrid smell, playing a significant role in our planet’s atmosphere and our daily lives. NO₂ comes from various sources, including car engines, power plants, and even from lightning during thunderstorms. It’s a part of a bigger family of gases called nitrogen oxides, which are not only key players in forming smog and acid rain but also contribute to the nutrient cycle within ecosystems. Knowing about nitrogen dioxide helps us grasp the delicate balance of our environment and the impact human activities have on it. This gas teaches us the importance of clean air and the steps we need to take to protect our planet.
| Property | Value |
|---|---|
| Formula | NO₂ |
| Name | Nitrogen dioxide |
| IUPAC Name | Nitrogen dioxide |
| Alternate Names | nitrito, nitro, nitrogen oxide |
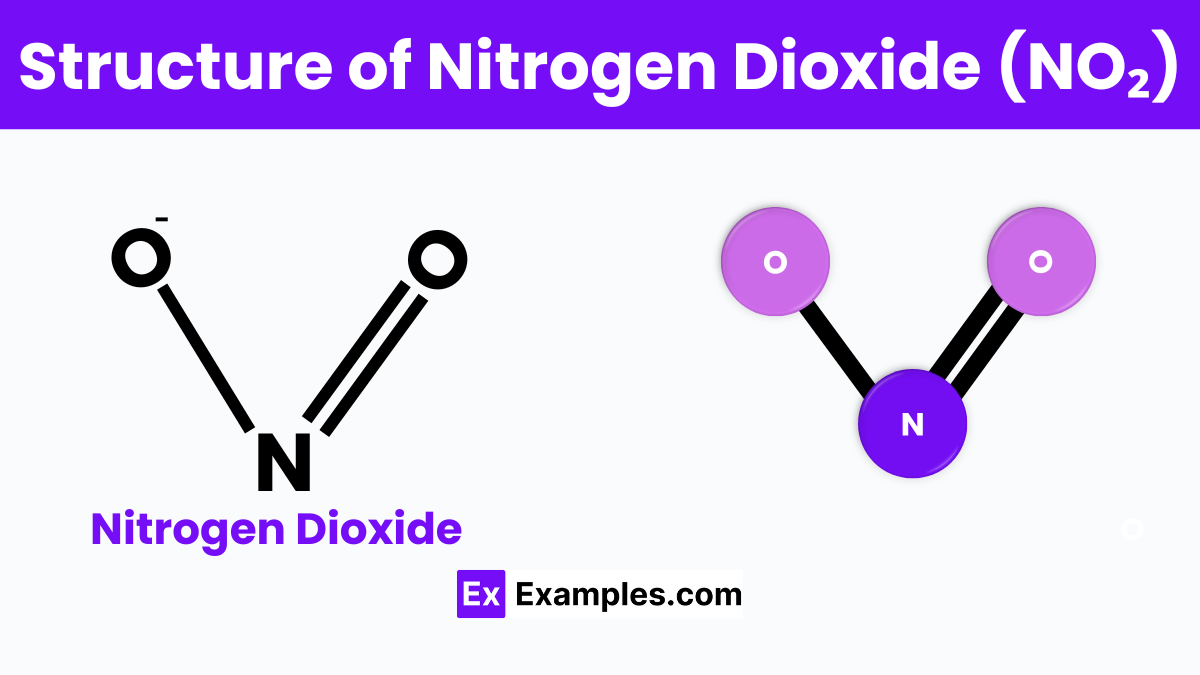
Nitrogen dioxide, often symbolized as NO2, is like a tiny molecule puzzle with one nitrogen atom holding hands with two oxygen atoms. Imagine the nitrogen atom in the center, acting as a hub, and the two oxygen atoms on either side, kind of like a V-shape. This arrangement is not just random; it’s because of the way electrons, the tiny particles that orbit atoms, like to share space and bond together. This sharing creates a strong connection between the nitrogen and oxygen, making NO2 a stable, yet reactive, molecule in our environment. What makes nitrogen dioxide really interesting is that it has an odd number of electrons, which means it’s always looking to share or steal an electron from other molecules, leading to various chemical reactions, especially in our atmosphere. This tiny molecular structure, small but mighty, plays a big role in the chemistry of the air we breathe.
Creating nitrogen dioxide (NO₂) can seem like a bit of chemistry magic, but it’s actually based on simple reactions. One common way to make NO₂ is by reacting copper with nitric acid. When these two substances meet, they undergo a chemical reaction that releases nitrogen dioxide as a gas. The equation for this reaction looks something like this
In simple terms, three pieces of copper react with eight units of nitric acid to produce copper nitrate, water, and our main event, nitrogen dioxide gas. This process is a great example of how combining different elements and compounds can create something entirely new. It’s important to note that making nitrogen dioxide should only be done in a controlled, safe laboratory environment because NO2 is a potent gas that can be harmful to breathe. This process highlights the fascinating ways in which chemicals react to form new substances, showing the dynamic nature of chemistry.
| Property | Description |
|---|---|
| Appearance | Reddish-brown gas that can give the air a hazy look. |
| Smell | Has a sharp, biting odor that’s pretty distinct. |
| State at Room Temperature | Gas form, making it blend into the air we breathe. |
| Solubility in Water | Slightly soluble, meaning it can mix into water a bit, but not completely. |
| Density | Heavier than air, which means it tends to hang low to the ground. |
| Melting Point | Turns from gas to liquid at about -11.2°C (11.84°F). |
| Boiling Point | Becomes a liquid at 21.15°C (70.07°F) under atmospheric pressure. |
When NO₂ encounters water, it doesn’t just dissolve; it actively reacts to form nitric acid (HNO₃) and nitrogen monoxide (NO). This reaction is a critical component of acid rain formation. The reaction can be represented by the chemical equation: 3CO₂ + 2H₂O → 2HNO₃ + NO.
NO₂ can join with another NO₂ molecule to create dinitrogen tetroxide (N₂O₄), especially at lower temperatures or under pressure. This behavior is reversible, showing how NO₂ can exist in two forms, balancing between them based on the environment. This equilibrium is illustrated by: 2NO₂ ⇌ N₂O₄
As a potent oxidizing agent, NO₂ can steal electrons from other substances. This property makes it a critical player in combustion reactions, smog formation, and various atmospheric processes. Its role as an oxidizer highlights NO₂’s contribution to environmental pollution and chemical transformations in the atmosphere.
NO₂ has the ability to absorb ultraviolet (UV) light from the sun, influencing atmospheric chemistry and affecting Earth’s climate and air quality. This absorption is essential for understanding the balance of energy in our atmosphere and the impacts of pollutants on global warming and weather patterns.
| Property | Value |
|---|---|
| CAS Registry Number | 10102-44-0 |
| PubChem Compound ID | 3032552 |
| PubChem Substance ID | 24857797 |
| SMILES Identifier | N(=O)[O] |
| InChI Identifier | InChI=1/NO2/c2-1-3 |
| RTECS Number | QW9800000 |
| MDL Number | MFCD00085341 |
| Property | Value |
|---|---|
| NFPA Health Rating | 3 |
| NFPA Fire Rating | 0 |
| NFPA Reactivity Rating | 0 |
| NFPA Hazards | Oxidizing Agent |

One of the most important uses of NO is in producing nitric acid (HNO₃), a key ingredient in fertilizers and explosives. By reacting NO₂ with water, manufacturers create this powerful acid, fueling agricultural growth and various industrial processes.
NO₂ is crucial in the chemical industry as a precursor to many other chemicals. Its reactive nature makes it perfect for synthesizing a wide range of compounds, including plastics, dyes, and pharmaceuticals, showcasing its versatility in manufacturing.
In the aerospace field, NO₂ serves as an oxidizer for rocket fuels. Its ability to help burn fuel more efficiently makes it invaluable for launching spacecraft, highlighting its role in exploration beyond Earth.
Surprisingly, NO₂ also has a role in reducing pollution. It’s used in scrubbing systems to remove harmful pollutants from industrial exhaust gases, turning it from a pollutant into a pollution fighter.
Scientists use NO₂ to study and monitor the Earth’s atmosphere. Its presence helps researchers understand air quality, ozone formation, and the dynamics of climate change, making it a key indicator in environmental science.
NO₂ indirectly contributes to food coloring production. It’s involved in creating specific dyes that color our foods, demonstrating its unexpected role in the food industry.
Health Concerns: Breathing in NO2 can be harmful, especially for people with asthma or lung conditions. It can irritate the airways, making it harder to breathe and increasing the risk of respiratory infections. This is why clean air is so important for our health.
Environmental Impact: NO2 doesn’t just stop at affecting our health; it also plays a role in forming smog and acid rain. Smog can reduce visibility and harm wildlife, while acid rain can damage forests, lakes, and buildings. NO2’s ability to absorb sunlight also contributes to the warming of the Earth’s atmosphere, adding to climate change challenges.
Ozone Layer: Closer to the ground, NO2 can help form ground-level ozone, a key component of urban smog. However, this isn’t good for us or the environment, as it can lead to breathing problems, harm plants, and reduce crop yields.
Yes, nitrogen dioxide can irritate the respiratory system, exacerbate asthma, and reduce lung function, making it harmful, especially to those with respiratory conditions.
The primary source of nitrogen dioxide is the burning of fossil fuels by vehicles, power plants, and industrial facilities, contributing to air pollution.
Absolutely, nitrogen dioxide is a significant air pollutant that contributes to smog, acid rain, and the greenhouse effect, impacting air quality and health.
Indoor NO2 can come from gas stoves, heaters, and tobacco smoke, accumulating in poorly ventilated homes and impacting indoor air quality.
Improving ventilation, using air purifiers with HEPA filters, and reducing the use of gas-burning appliances can effectively lower NO2 levels indoors.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical formula of nitrogen dioxide?
NO
NO₂
N₂O
N₂O₄
What is the oxidation state of nitrogen in NO₂?
+1
+2
+3
+4
What color is nitrogen dioxide gas?
Colorless
Green
Red-brown
Yellow
Nitrogen dioxide is a major component of which environmental issue?
Global warming
Ozone layer depletion
Acid rain
Photochemical smog
Which of the following processes produces nitrogen dioxide?
Photosynthesis
Combustion of fossil fuels
Electrolysis of water
Fermentation
What is the primary health effect of nitrogen dioxide exposure?
Bone fractures
Respiratory problems
Skin irritation
Vision loss
What type of bond is present between nitrogen and oxygen in NO₂?
Ionic bond
Metallic bond
Covalent bond
Hydrogen bond
At what temperature does nitrogen dioxide become a liquid?
-11.2°C
-30°C
-50°C
0°C
Which of the following is a method to reduce nitrogen dioxide emissions?
Increasing fossil fuel use
Enhancing deforestation
Implementing catalytic converters
Reducing solar energy use
Nitrogen dioxide is an intermediate in the industrial production of which acid?
Sulfuric acid
Hydrochloric acid
Nitric acid
Acetic acid
Before you leave, take our quick quiz to enhance your learning!

